 17
17
The biopharmaceutical industry is evolving rapidly, with various platforms like nucleic acid drugs, antibody therapies, and gene therapies relying heavily on precise and optimized workflows. Among the critical tools in these production environments is the DNase assay kit, a core component for quality control and efficiency during manufacturing.
In this article, we explore how companies like Hzymes, which supplies a wide range of upstream enzyme raw materials and chemical substrates, can integrate DNase assay kits to streamline processes from plasmid template production to mRNA enzymatic synthesis, covering the entire IVT (in vitro transcription) process.
In high-throughput production, the integration of DNase assay kits must align with existing production protocols. Companies must ensure compatibility across various stages of the production line, from upstream plasmid production to downstream processes like co-transcriptional or enzymatic capping. Given that Hzymes is a leading supplier of materials for both major capping techniques, efficient assay kit integration is crucial.
To maintain streamlined operations, it’s essential to identify bottlenecks, particularly during assay setup and readout procedures, and adjust accordingly. Customization for batch or continuous production can further enhance workflow fluidity.
The high accuracy and reproducibility of DNase assays are central to maintaining the production of nucleic acid drugs and other biopharmaceutical products. Standardizing reagent preparation and handling ensures consistency across multiple batches, critical for large-scale operations like those conducted at Hzymes.
Minimizing variability in fluorescence detection, a key method in these assays, involves controlling environmental factors like temperature and pH. Calibration of fluorescence readers is vital to maintaining high assay sensitivity, ensuring precise quantification during nucleic acid and mRNA synthesis processes.
With high-throughput production environments, real-time monitoring and validation of assay performance become essential for sustaining production efficiency. Automated systems can help detect deviations in real-time, enabling quick adjustments and mitigating risks of contamination or enzyme degradation.
Hzymes can incorporate these automated tools to track the performance of their enzyme and chemical substrate kits, ensuring they meet stringent industry quality standards and regulatory compliance.
Scaling DNase assays for larger production volumes requires careful adjustment of reagent volumes and assay conditions. The ability to adapt these kits to high-volume processing environments is particularly relevant for suppliers like Hzymes, whose products are critical for nucleic acid drug development, where mRNA synthesis happens at a large scale.
Automation, including the use of liquid handlers and plate readers, can optimize workflow for robotic systems. Such systems ensure that enzyme and substrate delivery remain precise and consistent, a must for the robust production demands of cell and gene therapies.
Successful long-term integration of DNase assay kits requires a continuous improvement mindset. By regularly engaging production teams and analyzing assay performance trends, companies can optimize processes over time.
Training is also vital for sustaining high levels of performance, ensuring that production personnel are well-versed in handling and managing the DNase kits. Knowledge transfer protocols are equally important, particularly when onboarding new staff in fast-growing biopharmaceutical companies like Hzymes.
Hzymes has designed and developed DNase detection kit and RNase detection kit based on nucleic acid fluorescence substrate method.
After special sequence design, the probe can identify a variety of DNase & RNase respectively, and meet the detection needs of a variety of samples in a variety of scenarios. Compared with an imported brand (fluorescent probe method), the minimum detection limits of DNase and RNase detection kits were as low as 1/8.
At the same time, the kit has gone through complete methodological verification, and the quality control is guaranteed. To test buffers or materials commonly used in molecular experiments, Hzymes Kit interferes with fewer species. For some samples with interfering substances, it can be diluted with water and tested according to the actual use scenario
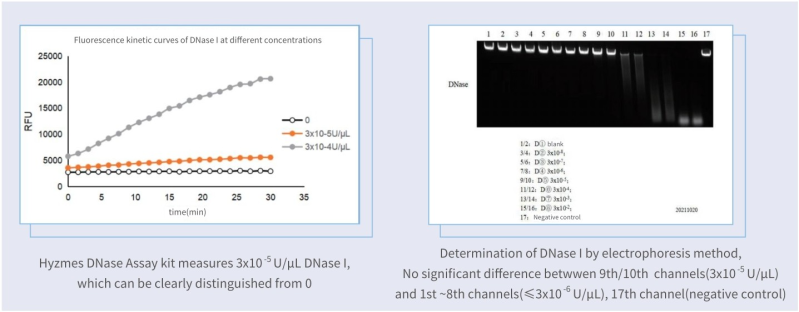
The Hzymes DNase Assay kit was in comparison with the nucleic acid hydrolysis-gel electrophoresis method, the same low-concentration enzyme samples were measured as shown in the figure:
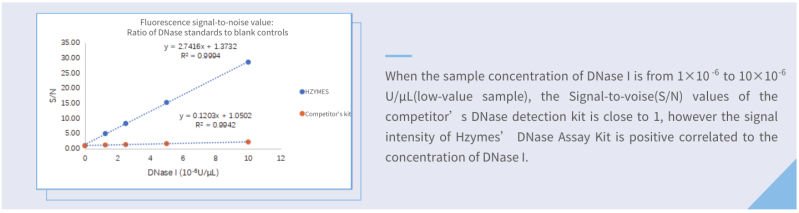
Compared with one of the best competitor kits (fluorescence), the limit of detection of Hzymes DNase Assay kit is only 1/8 of that of the competitor kit.


Composition of the Hzymes DNase Assay Kit
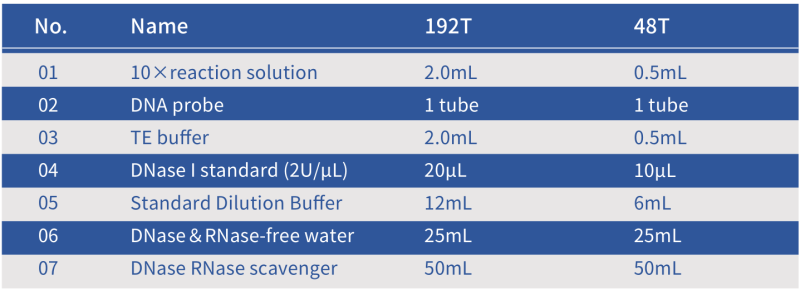
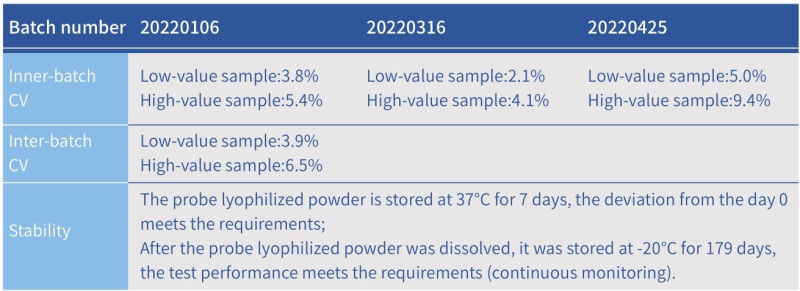
Note: The standard is DNase I. The unit for DNase I activity is defined as the amount of enzyme that completely degrades 1µg of pBR322 DNA in DNase I reaction buffer at 37°C for 10 minutes [1]; one DNase I activity unit is equivalent at 0.3 Kunitz units [2].
Product details

Conclusion
Optimizing the integration of DNase assay kits is a complex yet vital step in high-throughput biopharmaceutical production environments. Suppliers like Hzymes, with their extensive range of enzyme and substrate products, play a critical role in enabling this integration, particularly for applications ranging from mRNA synthesis to gene therapies. Continuous improvement and adaptation to high-volume production processes will ensure sustained efficiency and quality control in the future of biopharmaceutical manufacturing.

