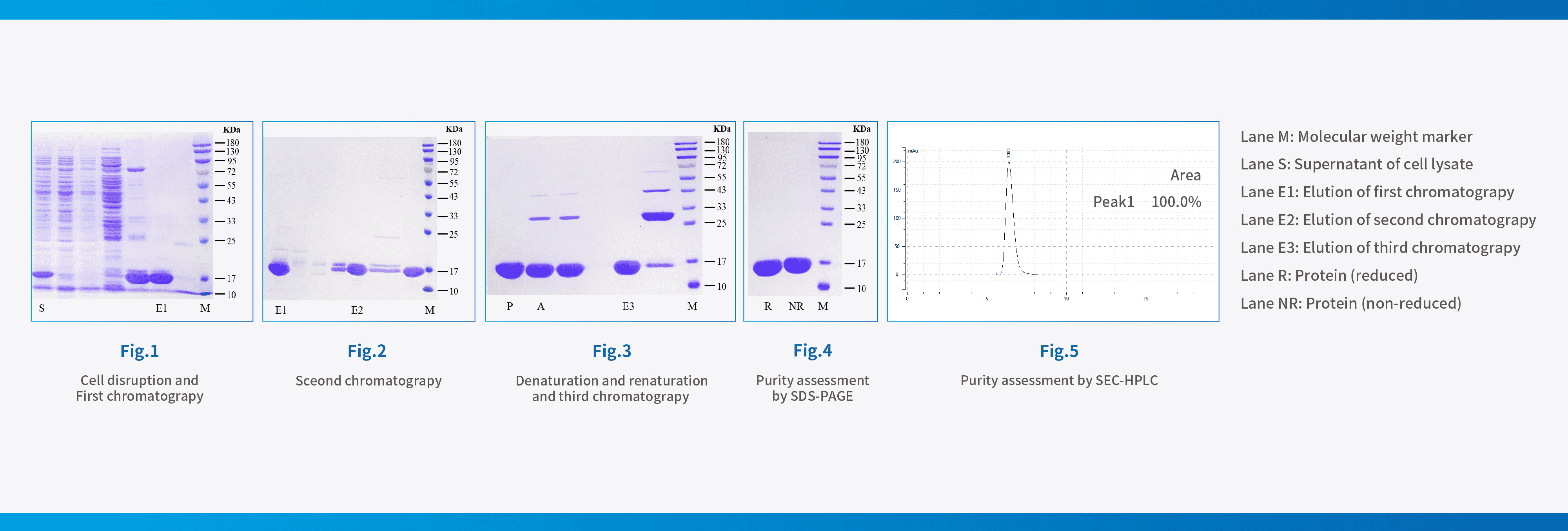
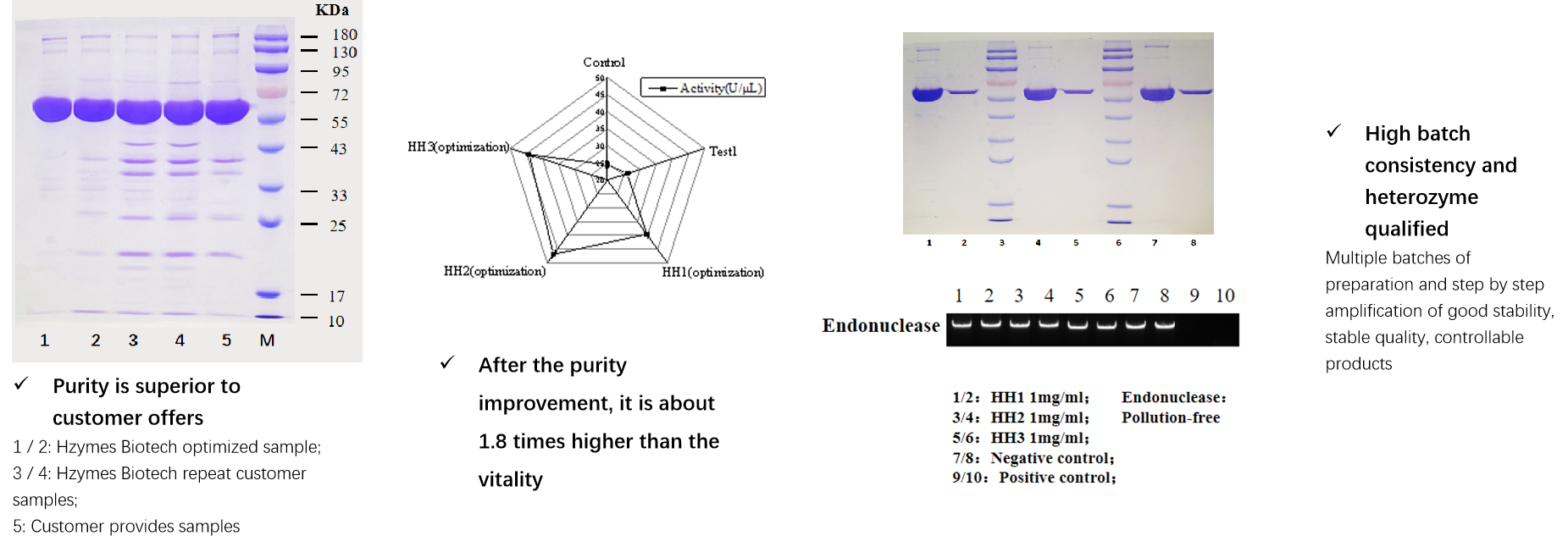
Case Description:A famous domestic molecular diagnosis company needs a special molecular enzyme raw materials, enzyme raw material sequence was known. However, the customer stated that there were difficulties in developing temperature control processes, scaling up processes, poor product stability, and limited mass production capacity during their own research and sample production processes, which directly affect the development of downstream testing reagents and supporting instruments. Therefore, Hzymes Biotech was entrusted to participate in the cooperative research, improve the process and implement the mass production of this raw material. After process optimization, the purity has been increased to more than 95%, about 1.8 times higher than the vitality. The consistency between batches is high, and the production capacity of a single batch has been increased by more than 4 times, which has been highly recognized by the customer.