 25
25
Glycosylation, the process of adding sugar molecules to proteins, is critical in biopharmaceuticals, as it significantly influences the structure, stability, and function of therapeutic proteins. N-glycans, a specific type of glycan, play a vital role in this process, affecting how proteins fold, their interactions with other molecules, and their overall therapeutic efficacy. Proper N-glycan profiling is essential because variations in glycosylation can impact a drug’s effectiveness, safety, and immunogenicity, making accurate glycan analysis a key factor in the development and quality control of biopharmaceuticals.
Challenges in N-Glycan Analysis stem from the inherent complexity of N-glycan structures, which consist of diverse and intricate branching patterns that can vary significantly between proteins and production batches. This structural diversity makes accurate analysis challenging, particularly in biopharmaceutical quality control, where precise glycan profiling is crucial for ensuring product consistency and efficacy. The difficulties include distinguishing between closely related glycan isomers, detecting minor glycan species, and quantifying glycan populations, all of which require advanced analytical techniques and robust methodologies. These challenges underscore the need for sophisticated tools and technologies to achieve reliable N-glycan analysis in biopharmaceuticals.
The Components of the N-Glycan Kit are meticulously designed to support accurate and efficient N-glycan analysis, essential for applications such as biopharmaceutical quality control. Central to the kit are Reagents and Enzymes, including key enzymes like PNGase F. PNGase F is critical for cleaving N-glycans from glycoproteins, a fundamental step in glycan analysis. The quality and purity of PNGase F directly influence the completeness of glycan release, thereby affecting the reliability of the analytical results. The kit also includes labeling dyes, such as 2-AB (2-aminobenzamide), which are used to fluorescently label the released N-glycans, significantly enhancing their detection sensitivity during analysis.
Sample Preparation Tools are another crucial component, offering a range of solutions for purifying and preparing glycans for analysis. These tools typically involve processes such as protein denaturation, glycan release, and cleanup to ensure that the sample is free from contaminants that could interfere with accurate glycan profiling. Proper sample preparation is essential for achieving reproducible and high-quality results.
The kit’s Analytical Instruments are designed to interface with various sophisticated platforms, including High-Performance Liquid Chromatography (HPLC), Mass Spectrometry (MS), and capillary electrophoresis. These instruments are necessary for the separation, identification, and quantification of the diverse glycan structures. Current trends in the field are pushing for increased instrument compatibility and automation, allowing for streamlined workflows and higher throughput. This is particularly important in high-demand settings like biopharmaceutical manufacturing, where consistent and accurate glycan analysis is critical to product safety and efficacy.
Advances in N-Glycan Analysis have been driven by significant Technological Innovations that enhance glycan detection and quantification. Recent advancements include high-resolution mass spectrometry (MS) and improved High-Performance Liquid Chromatography (HPLC) techniques, which provide greater sensitivity and precision in identifying and quantifying complex glycan structures. Additionally, the integration of Artificial Intelligence (AI) and Machine Learning (ML) into glycan data analysis is transforming the field. AI and ML algorithms can process large datasets quickly, uncover patterns and correlations, and predict glycan structures with high accuracy. These technologies streamline data interpretation and enable more efficient and comprehensive glycan profiling, accelerating research and development in glycoscience.
Applications of N-Glycan Kits are pivotal in Biopharmaceutical Development, where precise glycan analysis is crucial for the creation and optimization of biologics, such as monoclonal antibodies. N-glycans significantly influence the structure, stability, and function of these therapeutic proteins, impacting their efficacy, half-life, and potential immunogenicity. As a result, consistent and accurate glycan profiling is essential throughout the production process to ensure that these biologics meet the necessary therapeutic standards.
Moreover, regulatory agencies, including the FDA and EMA, require detailed characterization of glycosylation patterns as part of the approval process for biologics. N-Glycan Kits provide the tools needed to meet these Regulatory Requirements and Quality Control standards, enabling manufacturers to monitor and maintain glycosylation consistency across production batches. This ensures not only compliance with stringent regulatory guidelines but also the production of safe and effective therapeutic products.
Case Studies highlight the critical role of the N-Glycan Kit in biopharmaceuticals. For example, a pharmaceutical company used the kit to consistently monitor glycosylation patterns in monoclonal antibody (mAb) production, ensuring uniformity across batches and maintaining therapeutic efficacy. This approach was crucial for meeting regulatory standards and avoiding potential issues related to product variability. Additionally, in the development of a biosimilar, the kit was employed to closely match the glycan profile of the biosimilar with that of the original biologic, a key step in gaining regulatory approval. These real-world examples underscore the N-Glycan Kit’s importance in ensuring product consistency and regulatory compliance
Integration into Biopharmaceutical Workflows is streamlined with the N-Glycan Kit, designed for seamless compatibility with existing quality control (QC) processes. The kit easily integrates into automated systems, allowing high-throughput glycan analysis that aligns with the efficiency demands of biopharmaceutical production. Its protocols are optimized for robotic liquid handling, reducing manual errors and ensuring consistent results. Additionally, the N-Glycan Kit works well with advanced data analysis tools, including AI-driven platforms, enabling rapid interpretation of complex glycan data and facilitating real-time quality adjustments. This integration enhances both the precision and efficiency of glycan profiling within biopharmaceutical workflows.
The Hzymes N-Glycan Analysis Kit is a comprehensive tool designed for the analysis of N-glycans in monoclonal antibodies and various N-linked glycoproteins. This product is tailored to meet the growing demand for “fast” labeling and mass spectrometry (MS) sensitivity, allowing researchers and scientists to delve deeper into the world of protein glycosylation.
Product Feature
Product Formation
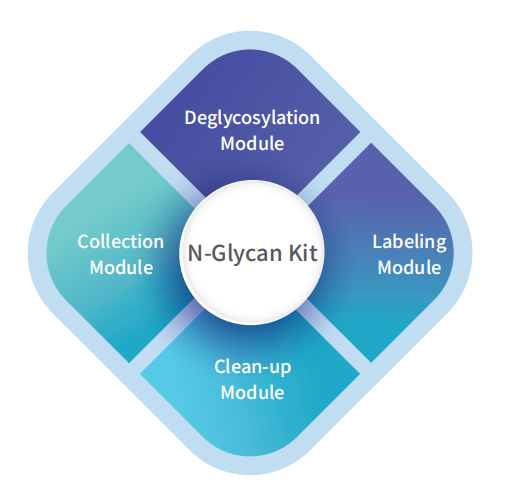
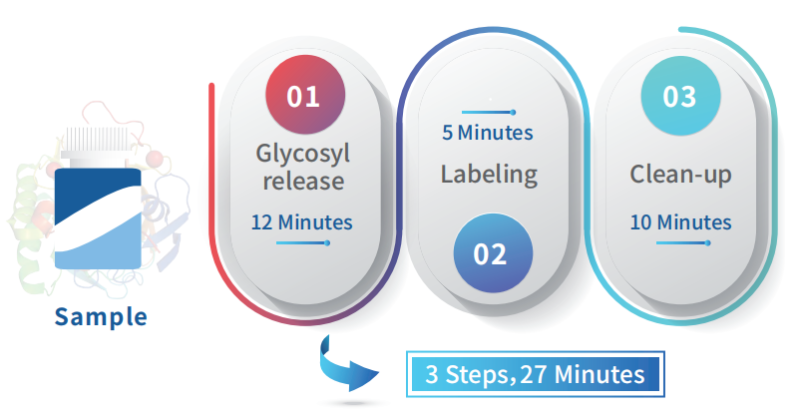
Operation Process
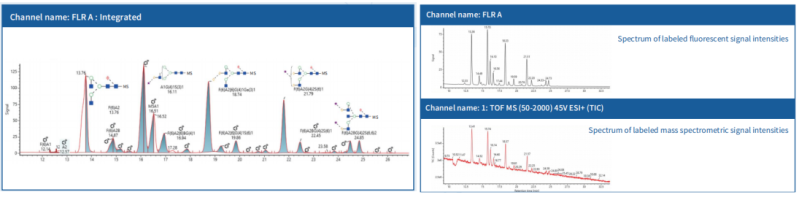
Spectrum of Labeled Molecular Weight
Hzymes N-Glycan Analysis Kit is a game-changing tool for researchers and scientists involved in the analysis of N-glycans in monoclonal antibodies and N-linked glycoproteins. With its four streamlined modules, user-friendliness, and flexibility in sample quantity, this kit promises to accelerate the pace of glycan analysis while maintaining the highest standards of accuracy and sensitivity. By utilizing this kit, researchers can unlock the secrets of glycosylation and contribute to advancements in biological research and pharmaceutical development.
Conclusion
N-Glycan Kits are essential tools in biopharmaceutical development, playing a crucial role in ensuring the consistency, efficacy, and safety of therapeutic proteins through accurate glycosylation analysis. Their applications span from monitoring monoclonal antibodies to demonstrating biosimilarity in biosimilars, making them vital for meeting regulatory requirements. Looking ahead, the future of glycan analysis is set to be shaped by advancements in detection technologies and the integration of AI and machine learning, which will further enhance the precision and efficiency of glycan profiling, driving innovation in biopharmaceutical research and quality control.

