Optimizing High-Sensitivity Detection of T7 RNA Polymerase Activity Using ELISA
T7 RNA polymerase plays a pivotal role in biopharmaceutical research, especially in the context of in vitro transcription and mRNA synthesis. This enzyme is widely used to generate high yields of RNA transcripts, making it a cornerstone in the production of mRNA-based therapeutics, vaccines, and diagnostic tools. Its efficiency and specificity are essential for producing the high-quality RNA required for these applications. Accurate measurement of T7 RNA polymerase activity is therefore critical, as it directly impacts the reliability and efficacy of RNA-based products. Ensuring precise enzymatic activity is crucial for the successful development and commercialization of cutting-edge biopharmaceuticals.
Technical Focus
- Principle of T7 RNA Polymerase ELISA:
The T7 RNA polymerase ELISA kit operates on the principle of enzyme-linked immunosorbent assay (ELISA), leveraging the specificity of antibodies to detect T7 RNA polymerase with high precision. In this assay, antibodies that are specific to T7 RNA polymerase bind to the enzyme, forming a complex. This complex is then detected using a secondary antibody conjugated to an enzyme, often horseradish peroxidase (HRP), which catalyzes a colorimetric reaction upon substrate addition.
The intensity of the resulting color is proportional to the amount of T7 RNA polymerase present, providing a quantitative measure through signal amplification. The assay is highly sensitive, capable of detecting low levels of T7 RNA polymerase, and exhibits exceptional specificity, distinguishing T7 RNA polymerase from other RNA polymerases, ensuring accurate and reliable results in biopharmaceutical research.
- Optimization of Assay Conditions:
Optimizing assay conditions for the T7 RNA polymerase ELISA involves fine-tuning critical parameters like buffer composition, incubation times, and substrate concentrations to enhance performance and reliability. Buffer composition plays a crucial role in maintaining the enzyme’s stability and ensuring proper antibody-antigen interactions, while carefully adjusted incubation times allow for optimal binding and reaction kinetics. Substrate concentration must be balanced to achieve strong signal generation without saturation, which could compromise quantification.
Common challenges in assay optimization include background noise, which can obscure true signals. To address this, thorough washing steps and the use of blocking agents can minimize nonspecific binding, ensuring clearer results. Additionally, adjusting the antibody dilutions and fine-tuning the enzyme-substrate reaction time can further reduce background interference, leading to more accurate and consistent assay outcomes.
- Application in RNA Therapeutics Development:
The T7 RNA polymerase ELISA kit is a valuable tool in the development of RNA therapeutics, particularly in high-throughput screening processes. It enables rapid and efficient identification of promising RNA candidates by allowing researchers to quickly measure and compare the activity of T7 RNA polymerase across numerous samples. This accelerates the discovery of effective RNA-based therapeutics.
Additionally, the assay plays a crucial role in quality control during mRNA production, especially in Good Manufacturing Practice (GMP) settings where consistency and accuracy are paramount. By providing precise measurements of T7 RNA polymerase activity, the ELISA kit ensures that mRNA is consistently produced at high quality, which is vital for the reliability and safety of vaccines and other RNA-based products in clinical and commercial applications.
- Comparative Analysis:
The T7 RNA polymerase ELISA kit offers distinct advantages over other detection methods like qPCR and Western blotting in the context of sensitivity, ease of use, and cost-effectiveness. While qPCR is highly sensitive and quantitative, it requires complex instrumentation and expertise, making it less accessible for routine applications. Western blotting, though specific, is labor-intensive and less quantitative, often requiring significant time and resources.
In contrast, the ELISA kit is user-friendly, cost-effective, and provides robust quantitative data with high sensitivity, making it ideal for high-throughput applications. Several case studies have demonstrated the critical role of the T7 RNA polymerase ELISA in advancing biopharma projects. For instance, in one study, the ELISA kit was instrumental in optimizing mRNA production processes for a novel vaccine, ensuring consistent enzyme activity and enabling the successful scale-up of production under GMP conditions. This facilitated the rapid development and approval of a life-saving therapeutic, showcasing the ELISA kit’s impact on biopharma innovation.
Hzymes Biotech T7 RNA Polymerase ELISA kit
Simple and Rapid One-Step Elisa for T7 RNA Polymerase Detection
This kit detects the residual amount of T7 RNA polymerase by double antibody sandwich enzyme-linked immunosorbent assay (ELISA) in accordance with the recommended method in the “mRNA Vaccine Quality Analysis Method – Draft Guidance”.
Advantages Highlight
- Easy Operation:
one step protocol with sample-to-result in 75min.
- Excellent Adaptability:
Standard Curve: R 2> 0.99.
Detection Limit <0.1 ng/ml.
Limit of Quantification: 0.25 ng/ml.
High accuracy and Repeatability: CV <10% with spike recovery in the range of 80% -120%.
- High Specificity
No cross-reactivity to other proteins.
- Good Adaptability:
Adapt to different T7 RNA polymerase from multiple suppliers.
Performance Data
- Limit of Detection (LOD) as low as 0.1ng/ml and Limit of Quantification (LOQ) as low as 0.25 ng/ml:
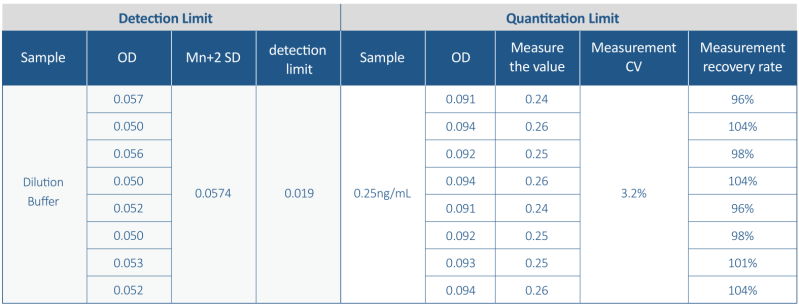
2. High accuracy and Repeatability: CV <10% with spike recovery in the range of 80% -120%.
Repeatability and Accuracy

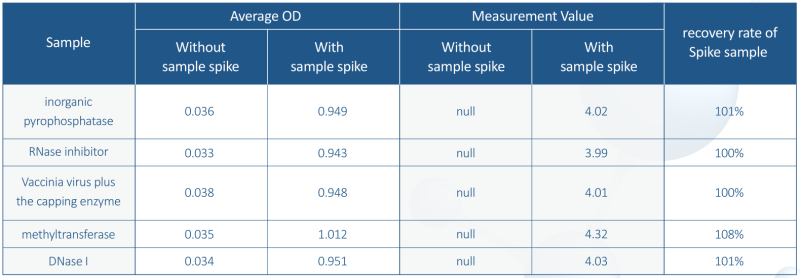
3. High Specificity: no cross-reactivity to other proteins
The T7 RNA polymerase Elisa kit only specifically recognizes the T7 RNA polymerase with no cross-reactivity to other IVT tool enzymes.
4. Good Adaptability:
Current T7 RNA Polymerase ELISA kit from suppler A, B, C, D doesn’t match with multiple T7 RNA polymerase on the market.

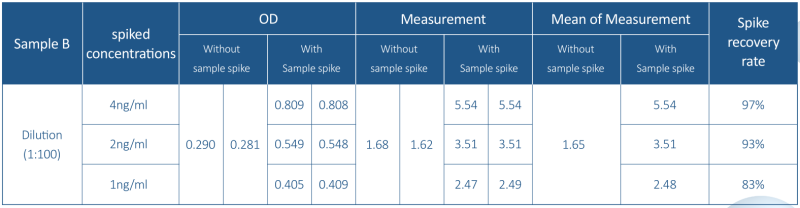
5. mRNA Sample Measurement
T7 RNA polymerase residues detection was performed for actual mRNA samples with the concentration 1-2mg/ml obtained from different CMC process stages.
Note: Sample B was spiked at the maximum dilution factor of 4ng/ml, 2ng/ml, and 1ng/ml, respectively. Samples A and C are in each the dilution factor was spiked at 4ng/ml.



6. Good Reproducibility: Mouse Epo mRNA sample at 1 mg/ ml concentration was measured for 3 times

How to Order

Conclusion:
The T7 RNA polymerase ELISA kit significantly enhances biopharmaceutical research by providing a reliable and efficient method to monitor and optimize RNA polymerase activity. This capability is crucial for the development of RNA-based therapies, as it ensures the production of high-quality mRNA, thereby accelerating innovation in vaccines, therapeutics, and diagnostics. The kit’s ease of use, sensitivity, and specificity make it an indispensable tool in the biopharma industry, supporting both high-throughput screening and rigorous quality control in mRNA production. Looking forward, advancements in ELISA technology, such as multiplexing capabilities and integration with automation, could further streamline biopharma applications, enabling even faster and more precise development of RNA-based products. These innovations hold the promise of expanding the impact of ELISA in the rapidly evolving field of RNA therapeutics.











Please first Loginlater ~