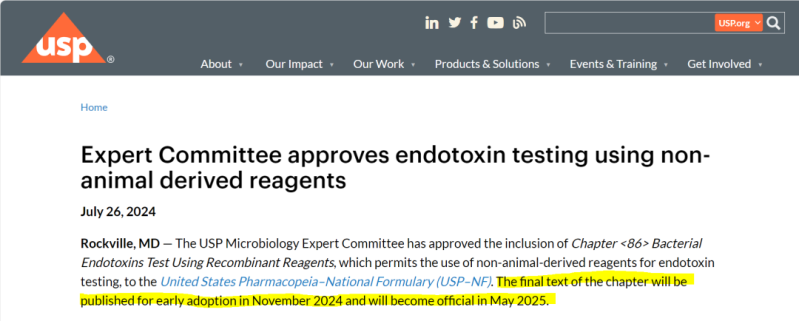
 62
62
The United States Pharmacopeia (USP) Expert Committee approved the inclusion of USP<86> Bacterial Endotoxins Test Using Recombinant Reagents in the “United States Pharmacopeia-National Formulary” on July 26, 2024. The USP<86> chapter will be published in November 2024 and will officially take effect in May 2025.
Future Drug Testing
Limulus Amebocyte Lysate (LAL) reagent is a reagent derived from the blood cells of horseshoe crabs, relying on natural resources. In March 2019, the International Union for Conservation of Nature (IUCN) classified the Chinese horseshoe crab as endangered.
With the increasing variety of bacterial endotoxin tests year by year, the demand for LAL reagents in various countries has also been increasing. The conflict between the demand for LAL reagents and the conservation of horseshoe crab resources has promoted the development of supplementary alternative methods for endotoxin testing. Endotoxin content must be tested in biologics, injectable drugs, chemical drugs, radiopharmaceuticals, antibiotics, vaccines, mRNA and plasmid drugs, cell therapy and gene therapy, and medical devices (such as disposable syringes and implantable biomaterials) and is used as a quality standard for final product release.
Endotoxin Detection Methods
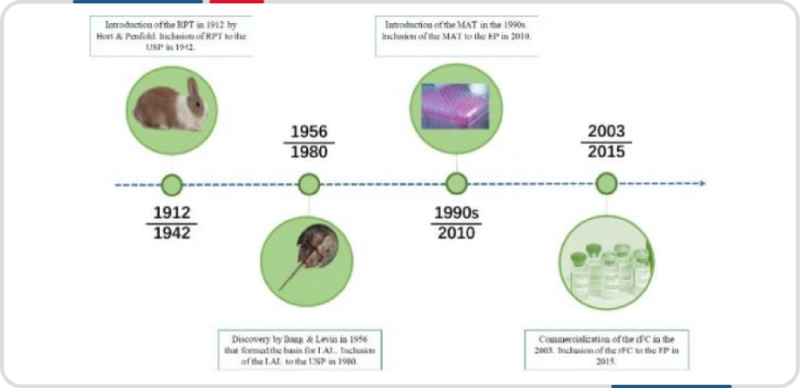
There are four main methods for detecting endotoxins: Rabbit Pyrogen Test (RPT), Monocyte Activation Test (MAT), Limulus Amebocyte Lysate (LAL) test, and Recombinant Factor C (rFC) test.
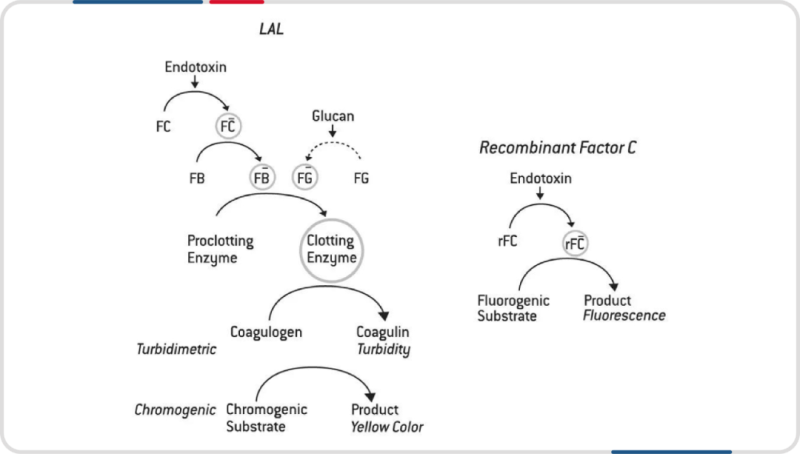
With the formal inclusion of recombinant cascade reagent (rCR) and recombinant factor C reagent (rFC) in Chapter <86> of the United States Pharmacopeia-National Formulary (USP-NF), pharmaceutical manufacturers will be able to adopt more advanced and sustainable testing methods. This will improve the quality and safety of drugs while reducing reliance on animal-derived reagents. This change not only responds to global concerns about animal welfare but also reflects advancements in biopharmaceutical testing technology. This move will have a profound impact on global drug safety standards and lay a solid foundation for the future development of drug testing technologies.
New Product Release
Protect Endangered Animals, Reject "Horseshoe" Blood!
Accurate Endotoxin Measurement, We Have New Methods
Hzymes Biotech's Recombinant C Factor Endotoxin Detection Kit expresses C factor recombinant protein through gene recombination. The recombinant C factor is activated by binding to endotoxins, which then cleaves a fluorescent substrate to release free fluorescent groups. The release of fluorescent groups is proportional to the concentration of endotoxins, allowing quantitative detection of endotoxin content. Compared with LAL reagents, the Recombinant C Factor Endotoxin Detection Kit has better specificity, precision, accuracy, linear range, and quantitative limits, making it the future mainstream method for endotoxin detection.
Product Performance

