HZYMES Leads the Release of Three Group Standards to Guide Industry Development
Latest News
mRNA technology offers multiple advantages: safety, efficiency, short production cycles, low cost, adaptability to rapidly mutating viruses, dual immune response (humoral and cellular), strong immunogenicity, adjuvant-free compatibility, and suitability for various diseases. These features give mRNA technology immense potential in combating infectious diseases and developing novel therapies. As research into this technology deepens, mRNA quality control is becoming increasingly crucial and stringent.
Hzymes, a leading developer of mRNA raw materials and quality control kits, has established detection methods for three critical quality control (QC) projects: dsRNA quantification, DNase residue detection, and RNase residue detection. These methods, validated through extensive experiments and rigorous methodological verification, are already employed by numerous companies in research and production.
As a vice president unit of the China Food and Drug Enterprises Quality and Safety Promotion Association, Hzymes spearheaded efforts with leading domestic mRNA companies and renowned universities to develop and document quality control detection methods and standards for dsRNA quantification, DNase residue detection, RNase residue detection, and T7 RNA polymerase residue detection.
- Quantitative Detection of dsRNA Impurities in mRNA Vaccines and Therapeutics via ELISA (T/FDSA 0058-2024)
- Detection of DNase Residues in Biological Products via Nucleic Acid Fluorescent Substrate Method (T/FDSA 0059-2024)
- Detection of RNase Residues in Biological Products via Nucleic Acid Fluorescent Substrate Method (T/FDSA 0060-2024)
The three group standards , after a strict authoritative expert group project review, publicity and closing review, lasted several months, and were officially released on November 14, 2024.

On November 15, 2024, the China Food and Drug Promotion Association’s Vaccine and Biologics Quality Evaluation and Standards Professional Committee held the release conference for the group standard
Development Guidelines for (Cell) Immunotherapy Products Based on mRNA-LNP Technology (T/FDSA 0055-2024) in Shenzhen.
This standard outline technical routes, preparation processes, inspection methods, QC highlights, functional validation, and requirements for management, personnel, facilities, equipment, and materials needed for R&D and production. It aims to provide clear technical guidance and product specifications for research institutions and production enterprises.
Hzymes contributed to this standard, which incorporates their detection methods.



ELISA Method for dsRNA Quantification in mRNA Vaccines and Therapeutics(T/FDSA 0058-2024)
ELISA is the mainstream method for dsRNA quantification, recommended by the USP Analytical Procedures for Quality of mRNA Vaccines and Therapeutics (Draft Guidelines: 3rd Edition) and the Development Guidelines for (Cell) Immunotherapy Products Based on mRNA-LNP Technology.
Due to variations in dsRNA length and sequence in different mRNA solutions, special technical considerations are required when selecting dsRNA standards and antibodies. The ELISA Method for dsRNA Quantification in mRNA Vaccines and Therapeutics provides a comprehensive explanation, ensuring the scientific validity and accuracy of dsRNA ELISA detection. It also offers detailed guidance on principles, validation, reagents, materials, experimental procedures, and result analysis.
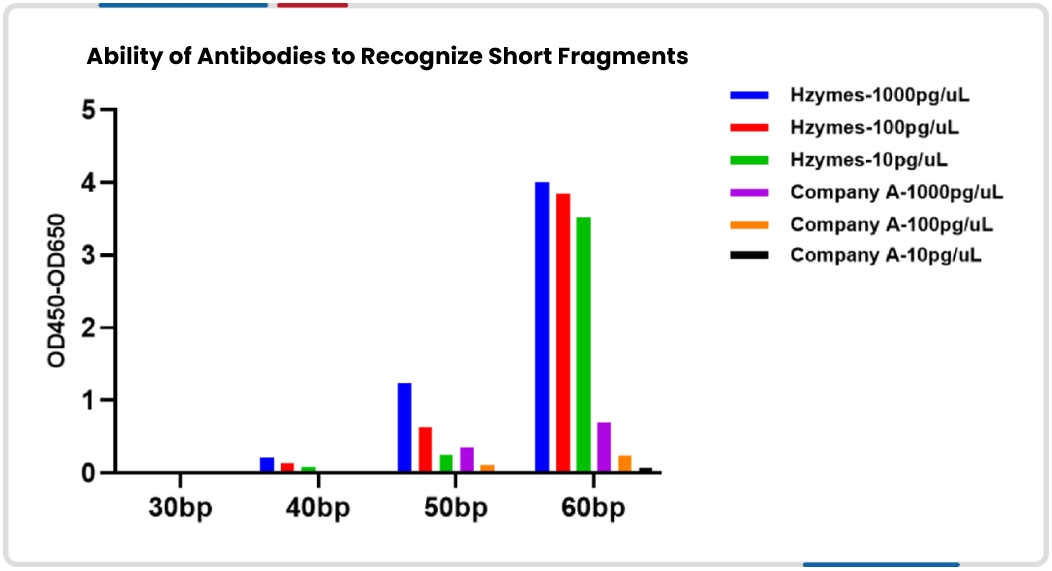
Detection of DNase/RNase Residues in Biological Products via Nucleic Acid Fluorescent Substrate Method(T/FDSA 0059-2024)/(T/FDSA 0060-2024)
During production, mRNA drugs and plasmid-based biologics may be contaminated by DNase/RNase residues, whether endogenous (from biological samples) or exogenous (from the environment, buffers, consumables, or raw materials). Such residues can trigger strong immunogenic responses and pose significant safety risks if they enter the human body. Therefore, accurate detection and control of DNase/RNase residues in external materials, consumables, and environments are necessary.
Hzymes developed a nucleic acid fluorescent substrate method for detecting DNase/RNase residues in biologics. This method addresses three major technical challenges:
- 1. Coverage of different DNase/RNase types.
- 2. Enhanced detection sensitivity.
- 3. Applicability to complex samples.
Based on their research and experience, Hzymes provides detailed explanations of the experimental principles, reagents, materials, and operational steps in the Detection of DNase/RNase Residues in Biological Products via Nucleic Acid Fluorescent Substrate Method.
Adhering to a philosophy of excellence and craftsmanship, Hzymes continues to support the development of QC technologies for biologics in China.
For more details, see the group standard announcement link:
https://www.ttbz.org.cn/StandardManage/Detail/116372/
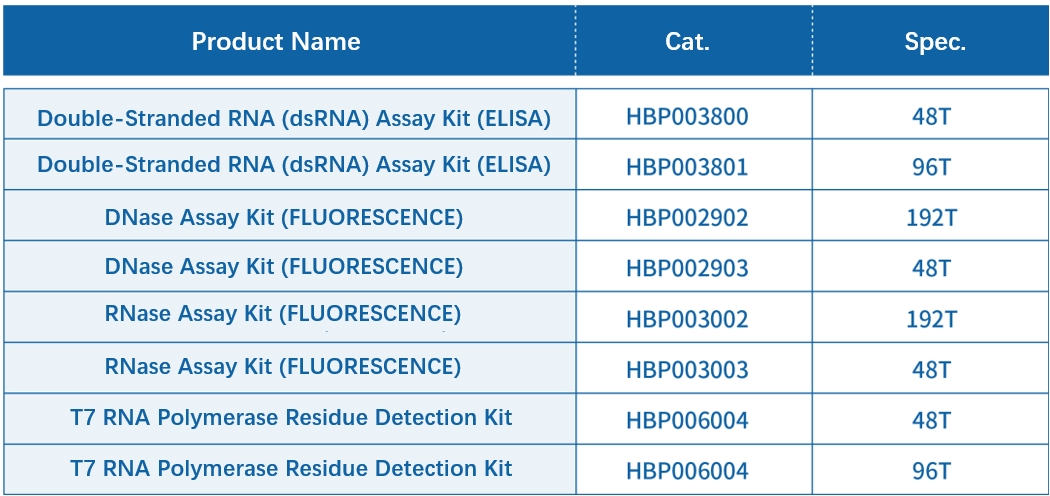
Recommended Products











Please first Loginlater ~