Application of mRNA in Veterinary Vaccines: PRRS Vaccine Research and Development
Background Overview
As mRNA vaccine and drug technologies mature, their applications are expanding, from the initial mRNA vaccines to various protein replacement therapies, cancer vaccines, cell therapies, and more. In addition to human vaccines and drugs, mRNA technology has shown significant potential in the field of veterinary vaccines.
Firstly, mRNA vaccines are developed relatively quickly. Traditional vaccine development usually takes years, whereas mRNA vaccines can be designed and produced in weeks. This is especially crucial for responding to emerging infectious diseases, like African swine fever or highly pathogenic avian influenza. Secondly, mRNA vaccines are highly safe, as they do not involve live or attenuated viruses. Lastly, mRNA vaccines are easier to scale up for production. Unlike traditional vaccines, they do not require complex biosafety facilities, allowing for rapid mass production during large outbreaks at a lower cost than traditional vaccines.
01 Lightweight mRNA
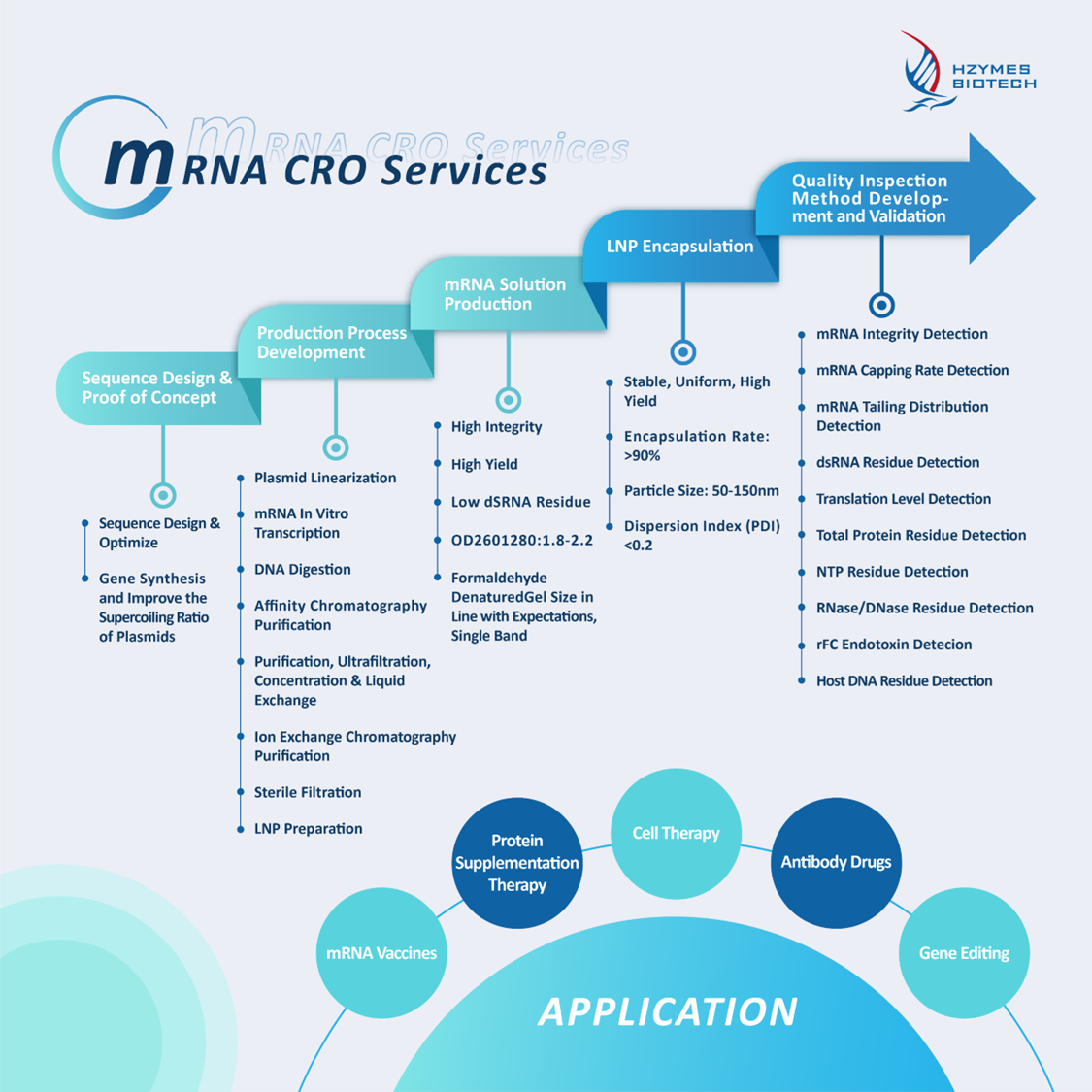
HZYMES has launched a range of services and products suited for early-stage research and scientific customers, simplifying the preparation process of research-grade mRNA/LNP. This approach meets the needs of early research clients who prioritize speed and cost, significantly reducing equipment and time costs during the preliminary research stage. This enables "zero-barrier" entry into the mRNA platform, contributing to the continuous development of the industry.

HZYMES was invited to exhibit at the 2024 (Shanghai) 9th Vaccine China Leaders Annual Conference, held on October 30-31. Dr. Li Liang, the Director of Nucleic Acid Drug Research at HZYMES, delivered a keynote report titled "Lightweight mRNA Drug Development Solutions and Progress in PRRS Vaccine Research," where he discussed HZYMES‘ mRNA technology platform and advancements in PRRS (Porcine Reproductive and Respiratory Syndrome) vaccine accessibility, template preparation, RNA purification, and delivery improvements. (Follow the official account and reply with "PRRS" for the latest materials.)

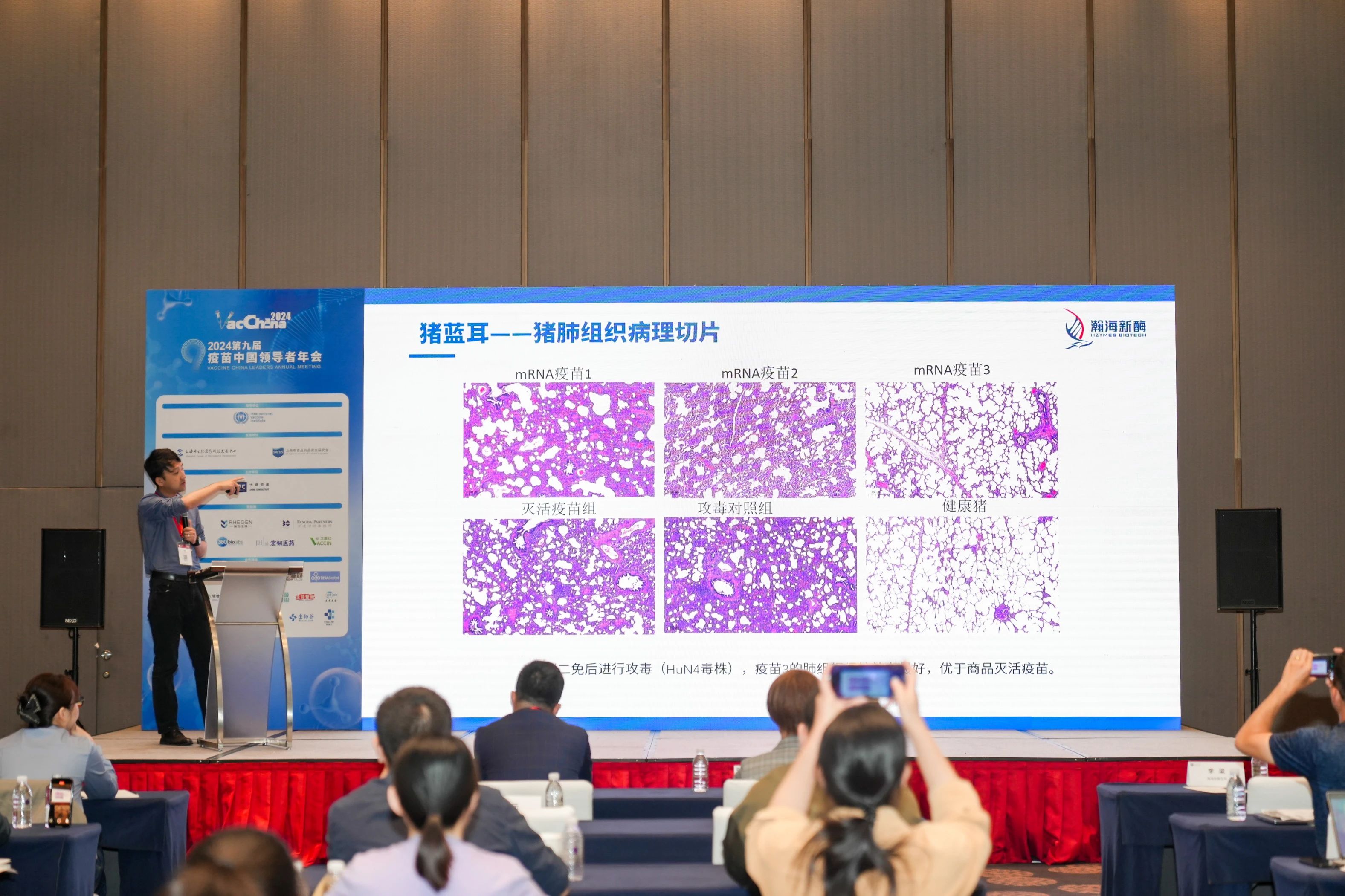
02 Application of mRNA in PRRS Disease
Porcine Reproductive and Respiratory Syndrome (PRRS): Commonly known as "blue ear disease," PRRS is caused by the respiratory syndrome virus (PRRSV), which leads to reproductive issues in adult sows and respiratory problems in nursing piglets, severely affecting the global swine industry. Current vaccines on the market struggle with PRRSV’s complex structure, mutation susceptibility, and difficulty in triggering effective cellular immunity, resulting in weak immunogenicity and inadequate protection. The benefits of mRNA vaccines—such as low development costs, fast production, dual activation of humoral and cellular immunity, and easy adjustment to tackle new pathogen variants—enhance the efficiency of PRRS virus vaccine development and production.
PRRSV particles are spherical, and to date, seven structural proteins have been identified: N protein (nucleocapsid), M protein, GP2a, GP2b, GP3, GP4, and GP5. PRRSV is divided into two genotypes: Type 1 (European, represented by the Lelystad strain) and Type 2 (American, represented by the VR-2332 strain). The genetic similarity between the two types is about 60%, with the predominant strain in China being the Type 2 American strain.
HZYMES analyzed the PRRSV Type 2 strain, optimized the sequence designs for antigens GP2A, GP3, GP4, and GP5, prepared the mRNA, encapsulated it in LNP for formulation, and conducted immunization and challenge tests on pigs. This process helped identify the optimal target or target combination for safety and protection effectiveness. Upon the secondary immunization and challenge, vaccines 1 and 3 showed better protective efficacy, with vaccine 3 outperforming the commercial inactivated vaccine.
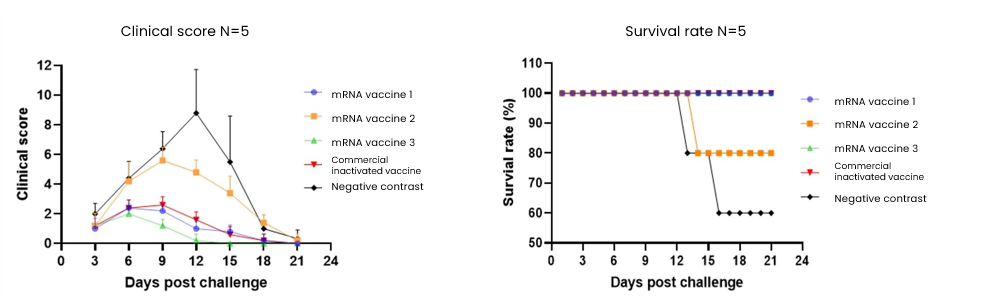
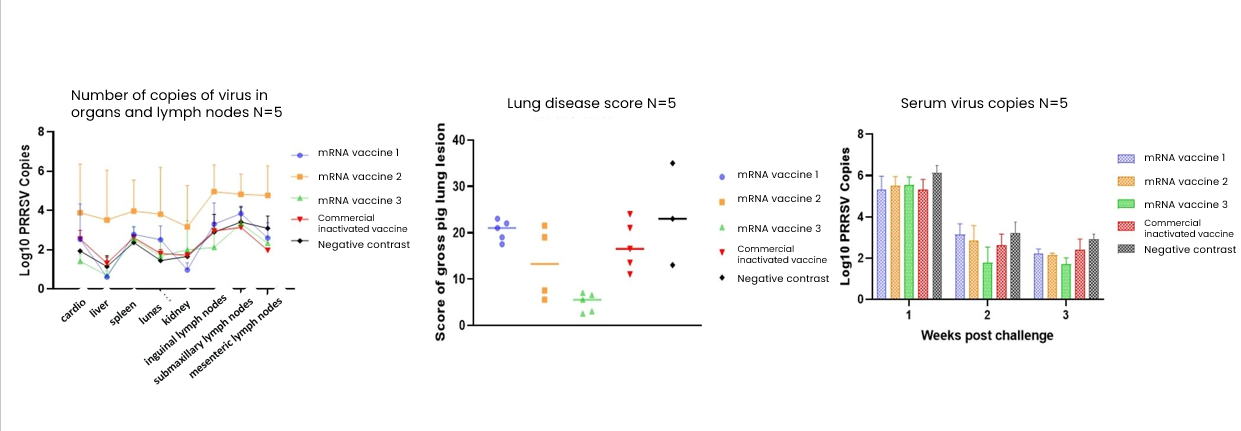
Conclusion
mRNA vaccine technology brings revolutionary changes to veterinary vaccines. Its rapid development, high safety, broad applicability, and scalable production make it an essential tool for future animal disease prevention.
With continuous technological advancements and expanded applications, mRNA vaccines will play an increasingly significant role in ensuring animal health and food safety. HZYMES, with its extensive range of high-performance materials and in-depth research on mRNA vaccines and drugs, offers comprehensive solutions for animal health.











Please first Loginlater ~