The Huge Potential of Nucleic Acid Vaccines Revealed at the Industry Conference!
Background Overview
Nucleic acid vaccines, as a new technological approach to vaccination, activate the immune system through specific pathways, enabling rapid immunity against a variety of diseases, including viral infections, tumors, bacteria, and parasites.
After years of development, nucleic acid vaccines have reached a pivotal moment—mRNA vaccines provide even greater imaginative possibilities for this field. The topic of "The Current Status and Future of the Chinese Vaccine Market" has increasingly garnered attention and discussion within the industry.
Industry Consensus
The focus and contemplation on nucleic acid drugs were highlighted at the "8th National Nucleic Acid Vaccine Conference," which recently concluded at the Suzhou Jinji Lake International Conference Center from September 19-22. At the event, Hzymes showcased a comprehensive range of mRNA products, host proteins/nucleic acids, quality control kits, vaccine process raw material enzymes, and CRO/CDMO services, among other latest offerings. Many professional researchers and companies were present to engage in in-depth discussions on the topic of "The Current Status and Future of the Chinese Vaccine Market," aiding in vaccine research and production.

Hzymes, through its self-established large-scale production system and high-standard pharmaceutical-grade specialty enzyme production framework, boasts a professional R&D team that offers: target sequence design, high-quality mRNA plasmid template preparation, IVT process optimization and validation, mRNA purification process development, LNP preparation process development and services, providing comprehensive product development support to facilitate the R&D of mRNA vaccine drugs.

Full-Series mRNA Platform Solutions
- mRNA Raw Materials: Driven by customer application needs, product innovation is prioritized. Hzymes has fully integrated innovative drug/vaccine research and production based on its core technology platform, providing GMP-compliant tool enzymes, high-purity synthetic substrates, and other complex high-end raw materials.
- GMP-compliant Raw Enzymes: A series of multifunctional T7 RNA polymerase variants have been developed, including high-specificity T7 RNA polymerase (HBP000330), low dsRNA T7 RNA polymerase (HBP000340), and heat-resistant T7 RNA polymerase (HBP000350), catering to different downstream RNA synthesis applications.
- High-Purity Synthetic Substrates: High-purity NTP substrates are available, along with a breakthrough patent-protected cap analog, both of which have completed DMF filing to expedite project submissions.
- In Vitro Transcription Kits: Utilizing optimized IVT systems for enzymatic capping and co-transcription capping, various types of RNA suitable for downstream applications can be rapidly and efficiently produced.
- mRNA Solutions: Supporting early-stage research and experimental controls, a variety of linear mRNA solutions, self-amplifying mRNA solutions, and customized demands are available.
- Quality Control Kits: Covering the entire mRNA quality control process. Hzymes has launched various kits, including dsRNA detection kits, T7 RNA polymerase detection kits, DNase and RNase detection kits, mRNA capping rate detection sample preparation kits, mRNA poly(A) tail length detection kits, host protein residue detection kits, host nucleic acid residue detection kits, DNA template residue detection kits, and recombinant C-factor endotoxin detection kits. This ensures quality control throughout the mRNA production process, guaranteeing the safety of mRNA products.
Hzymes is also working with mRNA companies to establish group standards in the mRNA field, promoting the industry's development.
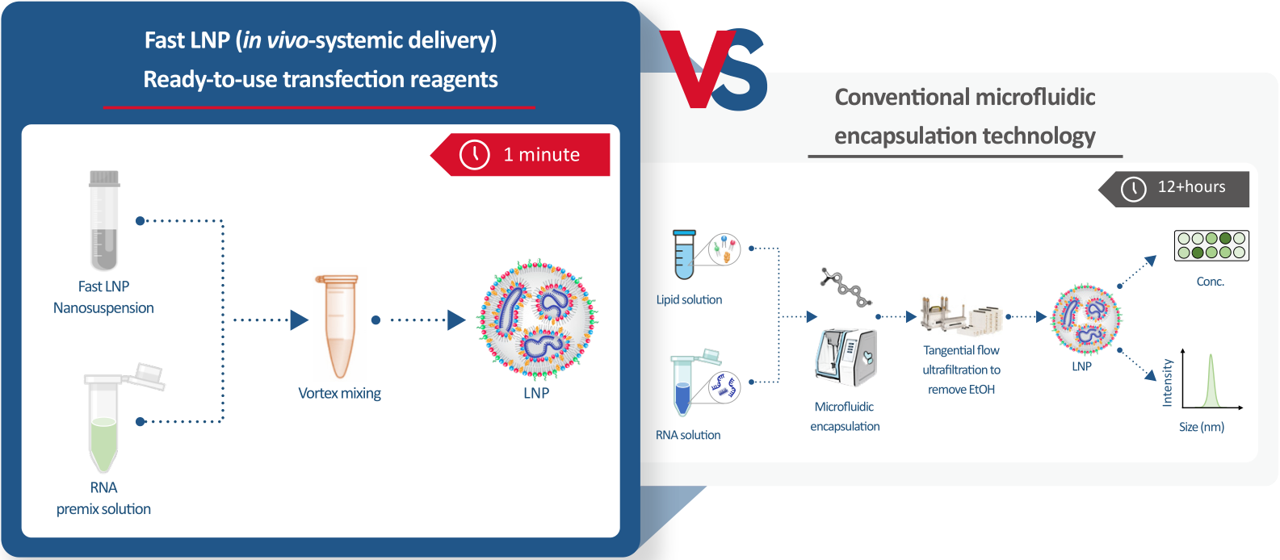
- Fast LNP: Rapid mRNA Encapsulation in 1 Minute. Traditional LNP preparation processes are complicated, requiring various equipment, which significantly raises costs and limits throughput, especially during early research or academic stages.
Hzymes has launched the Fast LNP rapid encapsulation kit: an off-the-shelf, in vivo delivery LNP—Fast LNP. Through hydrophobic interactions and intermolecular forces between lipid components, it can spontaneously assemble into mRNA-LNP with simple hand mixing, aiding quick target validation in early research or academic phases.
mRNA CRO Services
- One-Stop Service: Offering comprehensive solutions for mRNA vaccine and drug development and concept validation, accelerating early-stage research to commercial production.
- Rich Project Experience: Providing guidance for antigen design and mRNA sequence design.
- Comprehensive Quality Control Platform: Equipped with capillary electrophoresis, HPLC, CM, LC-MS, and other top-tier mRNA quality inspection platforms and supporting methods, with a robust quality management system ensuring strict control of mRNA product quality.
- Fast Delivery Cycle and Cost Control: Leveraging proprietary raw enzymes and extensive project experience, high-throughput screening systems provide project cycle and cost advantages for clients in CMC process development and optimization.
- Flexible and Open Technical Cooperation: Deep collaborations with CDMO partners meet diverse client needs at different stages.
Project Cooperation: Supporting Industrial Transformation
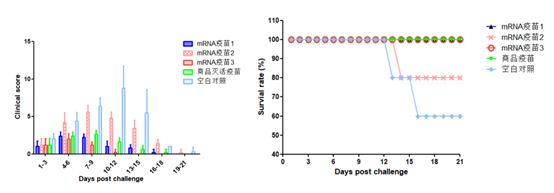
Leveraging core technological accumulation in the mRNA field, Hzymes has developed mRNA projects for porcine circovirus (PCV) and porcine reproductive and respiratory syndrome (PRRS), showing significant advantages in immunogenicity and longevity compared to traditional inactivated vaccines.
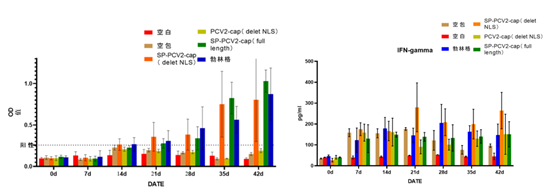 Porcine Circovirus (PCV) mRNA Project
Porcine Circovirus (PCV) mRNA Project
 Porcine Reproductive and Respiratory Syndrome (PRRS) mRNA Project
Porcine Reproductive and Respiratory Syndrome (PRRS) mRNA Project
We sincerely welcome collaborations based on professionalism and seek win-win partnerships to jointly advance the development of mRNA technology.











Please first Loginlater ~