Guidelines for the Development of (Cellular) Immunotherapy Products Based on mRNA-LNP Technology
Real-Time Information
On September 3, 2024, the “Guidelines for the Development of (Cellular) Immunotherapy Products Based on mRNA-LNP Technology” (T/FDSA 0055—2024) were officially released. The guidelines were jointly developed by more than 30 organizations, including businesses and public institutions, under the leadership of the Vaccine and Bioproduct Quality Evaluation and Standards Committee of the China Food and Drug Promotion Association. The standard will come into effect on October 3, 2024.
This important standard is China’s first general standard focused on the application of mRNA-LNP technology in the field of immunotherapy. It marks a milestone in China’s biopharmaceutical standardization efforts.

Source: Official announcement by the China Food and Drug Enterprises Quality Safety Promotion Association
The standard specifies the technical routes, preparation processes, testing methods, key quality control points, functional validation, and other aspects involved in the development of (cellular) immunotherapy products using mRNA-LNP technology. It also outlines the requirements for management, organization, personnel, facilities, equipment, and materials necessary for research and production. This provides clear technical guidance and product specifications for research institutions and manufacturers. The standard also sets specific requirements for product design under different technical routes and quality control indicators, aiming to drive continuous optimization and innovation in mRNA-LNP technology.

Source: Official website of the China Food and Drug Enterprises Quality Safety Promotion Association
The release of this standard fills a gap in the industry, laying a solid foundation for in-depth research and broad application of mRNA-LNP technology in the field of immunotherapy. It also provides clear, unified evaluation standards and technical guidelines for domestic and international peers, greatly promoting technological innovation and deepening international industrial cooperation.
Hzymes played a significant role in drafting this standard as one of the drafting organizations. The process involved multiple rounds of expert reviews and revisions, from the initial draft formed in December 2023, through continuous modification based on expert feedback, until the final draft was approved in July 2024. Every step of the process was the result of the dedication and wisdom of the drafting committee members.
Below is the previously released draft of the “Guidelines for the Development of (Cellular) Immunotherapy Products Based on mRNA-LNP Technology” for reference.
01: Your Essential Tool for Quality Control
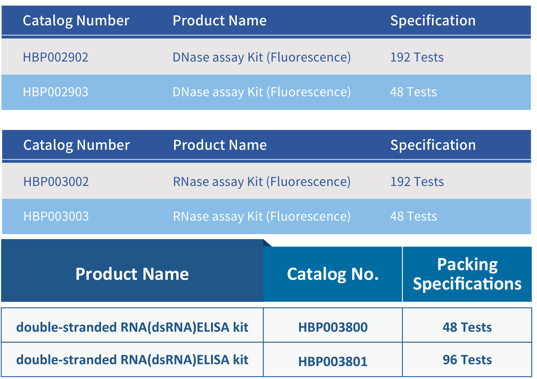
The National Drug Administration’s Key Laboratory for Vaccine and Bioproduct Quality Monitoring and Evaluation, together with Hzymes Biotechnology Co., Ltd., jointly developed three group standards: “Quantitative Detection of dsRNA Impurities in mRNA Vaccines and Drugs – ELISA Method,” “DNase Residue Detection in Bioproducts – Nucleic Acid Fluorescent Substrate Method,” and “RNase Residue Detection in Bioproducts – Nucleic Acid Fluorescent Substrate Method.” These standards provide detailed guidance on the quantitative detection of dsRNA, DNase residue detection, and RNase residue detection.
Hzymes dsRNA Quantitative Detection Kit
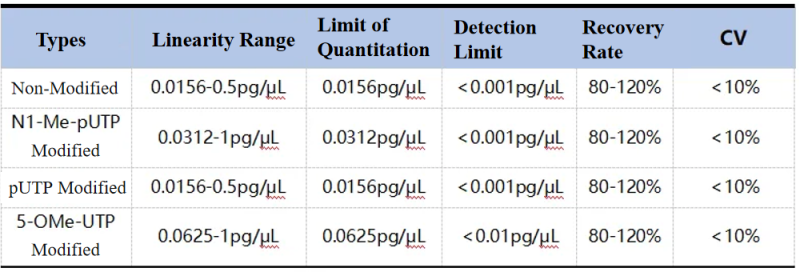
During mRNA production, T7 RNA polymerase transcribes from a DNA template, forming double-stranded RNA (dsRNA) byproducts of various lengths. These foreign dsRNA molecules can be recognized by cellular receptors such as Toll-like receptors (TLR), retinoic acid-inducible gene I (RIG-I), and melanoma differentiation-associated protein 5 (MDA5), which activate signaling pathways that release cytokines such as TNF-α and IFN-γ, leading to inflammation. On the one hand, dsRNA interferes with the feedback between the immune system and translation system, reducing the translation efficiency of mRNA; on the other hand, it triggers memory immune responses, reducing drug efficacy.
To monitor and control the residual impurities in vaccines, strict control over dsRNA levels in mRNA production is required.
Hzymes DNase/RNase Detection Kits
During the production of mRNA drugs and plasmids, there may be residual or contamination with DNase/RNase, including endogenous DNase/RNase from biological samples and external DNase/RNase from the environment, buffer solutions, consumable surfaces, and raw materials. If residual DNase/RNase enters the human body with biopharmaceutical products, it may trigger strong immunogenic responses, posing significant safety risks. Therefore, accurate analysis and detection of DNase/RNase residues in external materials, consumables, and the environment are necessary to ensure they are within safe limits.
- Meets detection needs for various scenarios and sample types: Probe sequences are specially designed to recognize multiple types of DNase and RNase. Suitable for testing bioproducts, nuclease-free consumables, and enzyme-free reagents.
- Higher sensitivity and stronger anti-interference performance.
- The kit has undergone comprehensive methodological validation.
- Stable quality with long-term supply: Intra-batch CV < 10%, inter-batch CV < 15%.
02: Comprehensive mRNA Solutions
Hzymes offers comprehensive mRNA production solutions, including plasmid preparation, IVT process development, mRNA purification, LNP encapsulation, and related quality inspection platforms. They provide customers with end-to-end solutions based on raw materials, helping accelerate new drug development.
For early-stage research customers, they offer CRO services, proof-of-concept services, third-party quality testing, and various finished mRNA liquid products. These products encode proteins such as reporter genes, target proteins, gene-editing proteins, and antigen proteins. They also provide a complete set of high-performance enzyme raw materials, chemical substrates, IVT kits, and quality control kits related to mRNA vaccines and drugs.











Please first Loginlater ~