Optimizing Nuclease Detection in Biopharmaceutical Manufacturing: A Comparative Evaluation of DNase/RNase Assay Kit Efficacy
Mechanistic Insights of DNase/RNase Assay Kit
DNase/RNase assay’s biochemical mechanism is meticulously designed to detect minimal nuclease activity, leveraging its heightened sensitivity to both DNases and RNases. The core of the assay involves a substrate that is highly specific to nucleases, enabling the detection of even trace amounts of enzymatic activity. The kinetics of enzyme-substrate interactions are finely tuned, allowing for rapid and precise cleavage of the substrate by DNases and RNases. This specificity ensures that the assay not only detects nuclease activity but does so with a high degree of accuracy, making it an invaluable tool for studying nuclease-mediated processes.
Application in Quality Control
The DNase/RNase Assay Kit plays a vital role in quality control during the manufacturing of gene therapies, mRNA vaccines, and other nucleic acid-based biopharmaceuticals. By ensuring that products remain free of nuclease contamination, the assay helps maintain product integrity, which is crucial for the safety and efficacy of these therapies. In biopharma settings, the assay has been successfully implemented to detect and mitigate contamination risks, with data showing its effectiveness in identifying even low levels of nuclease activity. This proactive approach reduces the potential for compromised products, safeguarding both manufacturing processes and patient outcomes.
Comparative Performance Metrics
A detailed comparison of various DNase/RNase assay kits reveals significant differences in sensitivity thresholds, false positive/negative rates, and overall reliability. Some kits demonstrate superior sensitivity, capable of detecting even the slightest nuclease activity, while others may exhibit higher rates of false positives or negatives, potentially compromising accuracy. Reliability also varies, with certain assays offering consistent performance across different conditions. When selecting the most appropriate assay kit, manufacturers should consider their specific needs—such as the required sensitivity for detecting contaminants—and align their choice with regulatory requirements to ensure compliance and product safety.
Technical Optimization Strategies
To optimize assay performance, careful attention should be given to sample preparation, buffer compositions, and detection methods. Using high-purity reagents and minimizing sample handling can reduce contamination risks, while tailored buffer compositions can enhance enzyme-substrate interactions, improving sensitivity. Selecting the most appropriate detection method, such as fluorescence or colorimetric readouts, can further refine assay precision. Potential sources of variability, such as inconsistent sample quality or environmental factors, should be mitigated by standardizing protocols and rigorously controlling experimental conditions. These strategies collectively ensure consistent and accurate results, enhancing the assay’s reliability in various applications.
Future Perspectives
Emerging technologies in nuclease detection, such as advanced biosensors and CRISPR-based tools, are poised to significantly enhance existing assay methodologies. These innovations offer the potential for seamless integration with traditional assays, providing heightened sensitivity and faster detection times. Ongoing research is focused on refining these technologies to further improve assay sensitivity while simultaneously reducing assay time, all without sacrificing accuracy. These advancements hold promise for more efficient and precise nuclease detection, paving the way for broader applications in biopharmaceutical quality control and other critical fields.
A Quality Control Tool Besides You
Hzymes has designed and developed DNase detection kit and RNase detection kit based on nucleic acid fluorescence substrate method.
After special sequence design, the probe can identify a variety of DNase & RNase respectively, and meet the detection needs of a variety of samples in a variety of scenarios. Compared with an imported brand (fluorescent probe method), the minimum detection limits of DNase and RNase detection kits were as low as 1/8.
At the same time, the kit has gone through complete methodological verification, and the quality control is guaranteed. To test buffers or materials commonly used in molecular experiments, Hzymes Kit interferes with fewer species. For some samples with interfering substances, it can be diluted with water and tested according to the actual use scenario
DNase Assay Kit Product Features
- High Sensitivity: Limit of Detection is as low as 1.25×10 U/μL (DNase I), which is only 1/8 of the best competitor kit.
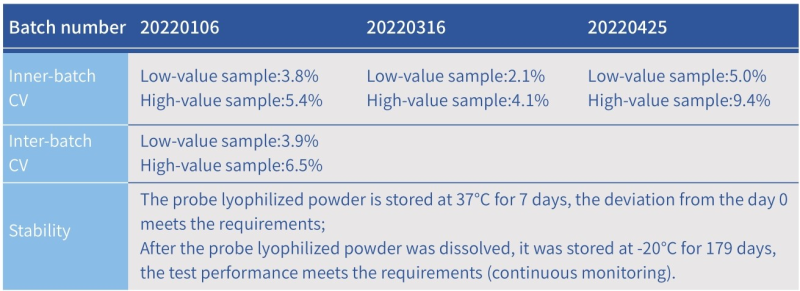
- High precision: intra-batch CV≤10%, inter-batch CV≤15%
- Good stability: valid for one year at -20℃. Once the probe solution is prepared, split and stored at -20°C.
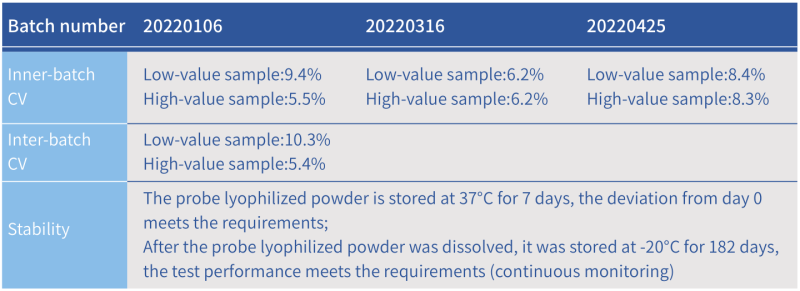
Precision and Stability of Hzymes DNase Assay Kit
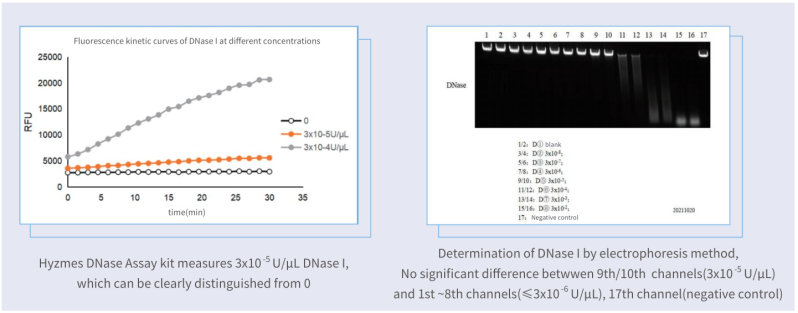
Sensitivity higher than gel electrophoresis method
The Hzymes DNase Assay kit was in comparison with the nucleic acid hydrolysis-gel electrophoresis method, the same low-concentration enzyme samples were measured as shown in the figure:
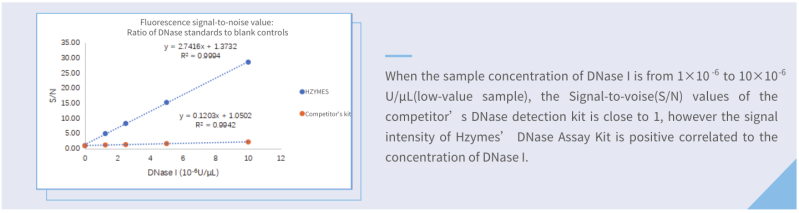
Sensitivity: Hzymes DNase Assay kit VS competitor kit
Compared with one of the best competitor kits (fluorescence), the limit of detection of Hzymes DNase Assay kit is only 1/8 of that of the competitor kit.


Composition of the Hzymes DNase Assay Kit
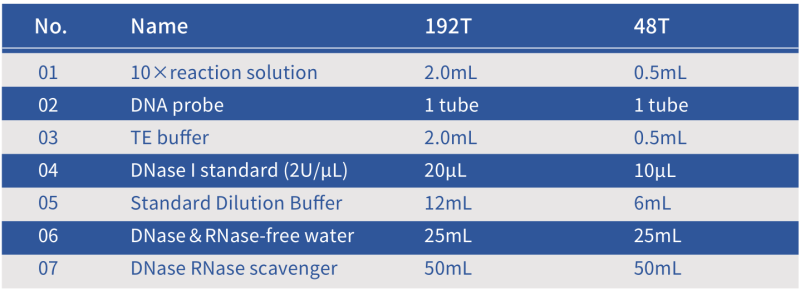
Note: The standard is DNase I. The unit for DNase I activity is defined as the amount of enzyme that completely degrades 1µg of pBR322 DNA in DNase I reaction buffer at 37°C for 10 minutes [1]; one DNase I activity unit is equivalent at 0.3 Kunitz units [2].

RNase Assay Kit Product Features
Characteristics of Products
- High sensitivity: the detection limit for RNase A is as low as 0.313pg/mL or about 1.56×10 U/μL which is only 1/8 of the best competitor kit.
- High precision: intra-batch CV≤10%, inter-batch CV≤15%
- Good stability: valid for one year at -20℃. After the RNA probe solution is prepared, aliquot and store at -20°C.
Precision and Stability of Hzymes RNase Assay Kit
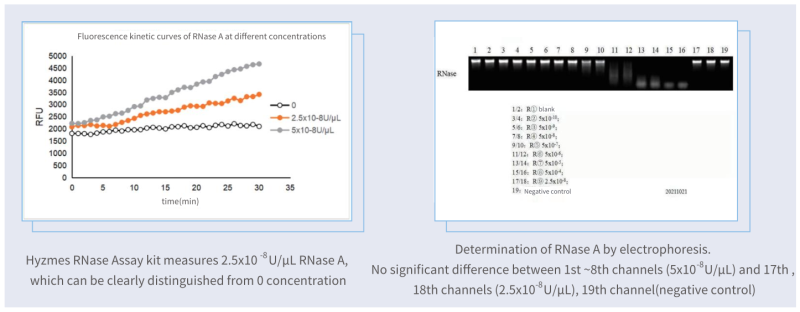
Sensitivity higher than gel electrophoresis method
The Hzymes RNase Assay kit was in comparison with the nucleic acid hydrolysis-gel electrophoresis method, the same low-concentration enzyme samples were measured as shown in the figure below.
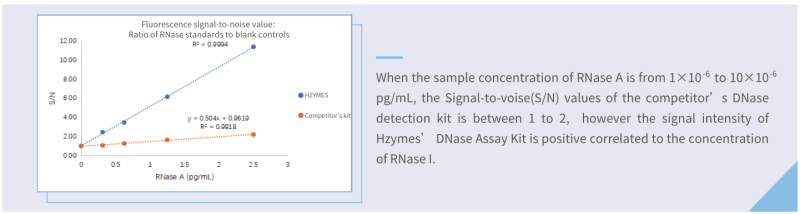
Sensitivity: Hzymes RNase Assay kit VS competitor kit
Compared with one of the best competitor kits (fluorescence), the limit of detection of Hzymes RNase Assay kit is only 1/8 of that of the competitor kit.


Composition of the Hzymes RNase Assay Kit
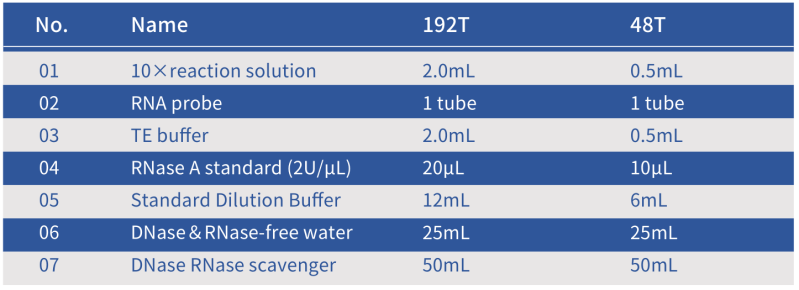
Note: The standard is RNase A. The unit for RNase A activity is defined as the amount of enzyme that increases 1.0 in the absorbance at 260 nm of yeast RNA at pH 5.0 and 37 °C [3], 50 RNase A activity units are equivalent to 1 Kunitz unit [4].
Product details

References
[1] New England Biolabs DNase I User Guide
[2] Kunitz M. Crystalline Desoxyribo – nuclease I. Isolation and General Pro – perties Spectrophotometric
Method for the Measurement of Desoxyribonuclease Activity[J]. The Journal of General Physiology, 1950, 33(4):349-362.
[3] Thermo scientific RNase A (DNase and Protease-free) User Guide
[4] Kunitz, M. A. A spectrophotometric method for the measurement of ribonuclease activity[J]. Journal of Biological Chemistry, 1946, 3(2):308-320.











Please first Loginlater ~