New Approach to Efficient mRNA Purification!
mRNA technology, as a new platform technology, is widely used in preventive vaccines, therapeutic vaccines, tumor treatment, immunotherapy, protein replacement, gene editing, and other fields. During in vitro transcription synthesis of mRNA, not only is the desired mRNA product produced, but also various impurities such as reaction enzymes (T7 RNA polymerase, inorganic pyrophosphatase), NTP, DNA templates, salt ions, and by-products (dsRNA, truncated RNA fragments) are generated.
These impurities can affect the accurate quantification of mRNA, reduce protein translation efficiency, and induce immunogenicity. Therefore, after in vitro transcription, purification is required to separate and purify the target mRNA from the IVT mixture, increasing the purity and integrity of the target product, ensuring the safety and effectiveness of the product.
Conventional mRNA purification methods mainly include: lithium chloride (LiCl) precipitation, magnetic bead purification, ammonium sulfate precipitation, and affinity chromatography purification. Among these, precipitation and magnetic bead methods are relatively simple and quick but are limited to small volumes, so they are mostly used in early research and development. In industrial production, chromatography purification is the main method, but it requires specialized purification equipment.
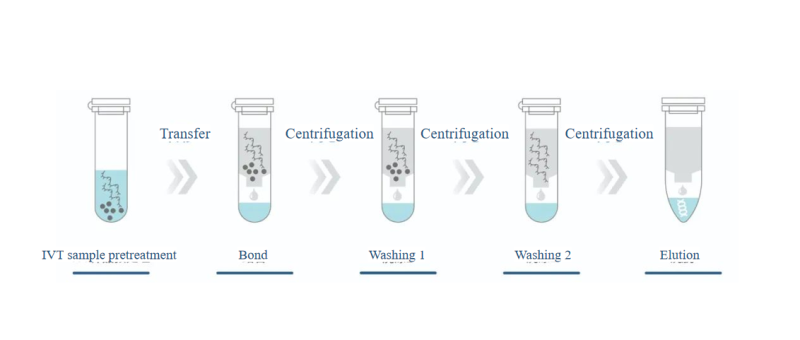
Introduction to Affinity Chromatography
The mainstream method of affinity chromatography is Oligo dT affinity chromatography. Oligo dT resin is based on polystyrene, with its surface covered by a large number of hydroxyl and functionalized poly(dT) groups. Under high salt conditions, salt ions shield the electrostatic repulsion between the negatively charged resin and mRNA, allowing the mRNA molecule and the Oligo dT column ligand to interact. The poly(A) tail of the mRNA pairs with the functionalized poly(dT) group to form hydrogen bonds, capturing the mRNA molecules. Impurities lacking the Poly(A) tail, such as free nucleotides, short transcription products, enzymes, etc., cannot bind to the Oligo dT column. When the buffer contains no salt, the negative charges on the phosphate groups of the dT ligand backbone and the poly(A) backbone cause electrostatic repulsion, which overcomes the hydrogen bonds, allowing the mRNA to be eluted efficiently with a yield of over 90%.
Traditional affinity chromatography requires specialized purification equipment, and the purification process needs constant optimization to meet industrial production demands. Now, a new lightweight affinity chromatography purification kit has been introduced, requiring only centrifugation for sample purification within 30 minutes. The purified mRNA can be used in various downstream experiments, enabling the rapid and convenient acquisition of high-quality mRNA.
Product Features
- High Efficiency and Flexibility: Suitable for RNA samples of various starting quantities, capable of purifying mg-level mRNA samples.
- Convenient and Economical: No need for purification equipment or low-temperature devices; simple operation saves equipment and labor costs.
- Good Reproducibility: The purified mRNA has high integrity, significantly removes NTPs, and can be purified within 30 minutes, meeting various experimental needs.
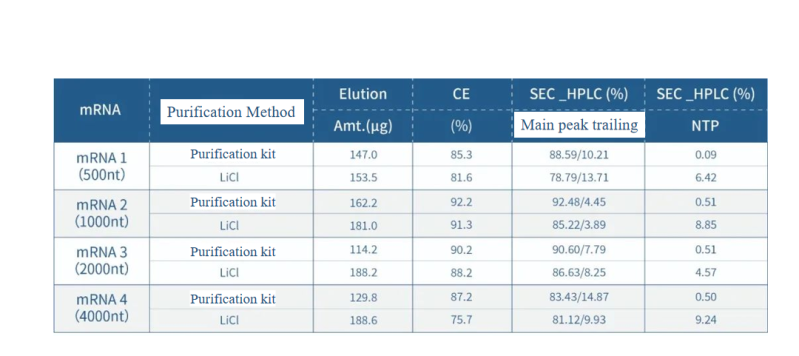
Performance Data Compared to the LiCl precipitation method, the affinity chromatography purification kit shows better results in improving CE integrity, SEC purity, and NTP removal.
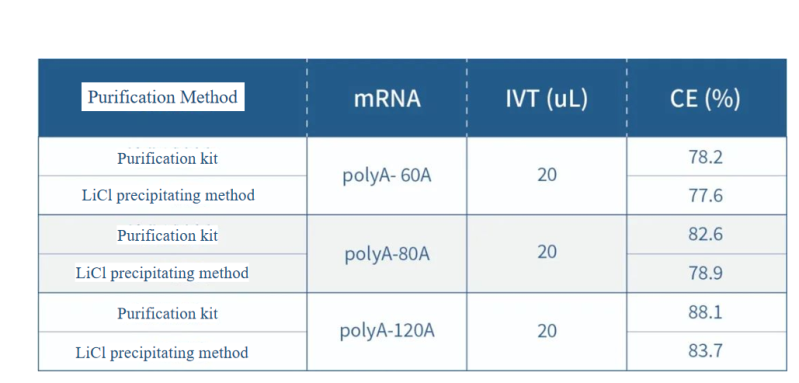
Comparison of mRNA fragments with different Poly A lengths using the self-developed purification kit and LiCl precipitation method: For the same mRNA fragment sequence, the integrity of different Poly A tail fragments is improved.
Bibliography:
[1] LiCl Precipitation for RNA Purification, Retrieved Jan 8, 2024.
[2] RNA Clean XP Performance and Data, Retrieved Jan 8, 2024.
[3] D Prazeres, T Schluep, C Coony, Preparative purification of supercoiled plasmid DNA using anion exchange chromatography, J Chromatogr A 806 (1998) 31–45.
[4] J Koubek, KF Lin, YR Chen, RP Cheng, JJT Huang, Strong anion-exchange fast performance liquid chromatography as a versatile tool for preparation and purification of RNA produced by in vitro transcription, RNA 19 (2013) 1449-1459.
[5] Xue Feng, Zhengjun Li, Zhiguo Su, Shiyi Che, Baiqian Dai, Yuan Cheng, Songping Zhang. Rapid and high recovery isolation of mRNA from in vitro transcription system by ammonium sulphate precipitation at room temperature. Separation and Purification Technology. Volume 336, 2024, 126331.











Please first Loginlater ~