Quantitative Detection of Residual Plasmid DNA: Who is the most Stable and Accurate Choice?
Background Overview
Plasmids are covalently closed, circular DNA molecules that exist independently of chromosomal DNA and replicate autonomously within bacterial and fungal cells. They typically carry a certain number of genes and are the most common vectors in genetic engineering.
The production process of mRNA drugs includes: seed template preparation, plasmid DNA fermentation, plasmid DNA purification, mRNA synthesis, mRNA purification, and LNP encapsulation purification and formulation filling. Among these steps, mRNA synthesis uses linearized plasmid DNA as a template for in vitro transcription. Therefore, there may be residual plasmid DNA in the mRNA bulk.
Residual plasmid DNA in biological products is not only an impurity introduced during production but also poses certain safety hazards. Once introduced into the human body, it can cause various outcomes, including carcinogenicity, infectivity, potential recombination risk, and increased immunogenicity. Measures should be taken in mRNA production to control and limit the amount of residual plasmid DNA, including process optimization, selection of raw materials, and enzymatic purification, to ensure the quality and safety of the product. To regulate the production process of mRNA drugs and ensure their quality stability and safety, both domestic and international regulatory agencies have established detection standards and methods for residual plasmid DNA.
Combining sensitivity, specificity, and short processing time, HanHai New Enzyme has developed a detection kit for residual plasmid DNA in mRNA drugs using the qPCR fluorescence probe method. This kit uses specific primers designed for the kanamycin resistance gene, enabling the quantitative detection of plasmid DNA residues containing the kanamycin resistance gene in samples.
Product Performance
Sensitive and Accurate: Capable of detecting plasmid DNA residues with high sensitivity and accuracy.
High Specificity: Primers are designed specifically for the kanamycin resistance gene, ensuring strong specificity.
Good Linearity: Offers a wide linear range and excellent linearity in standard samples.
Reliable Results: High accuracy and precision with stable sample recovery rates.
Wide Applicability: Suitable for various domestic and imported quantitative PCR instruments.
Stable Performance: Meets stability test standards, with the ability to be stored for extended periods.
Professional Methodology: Based on testing standards and methods established by domestic and international regulatory agencies, ensuring authority.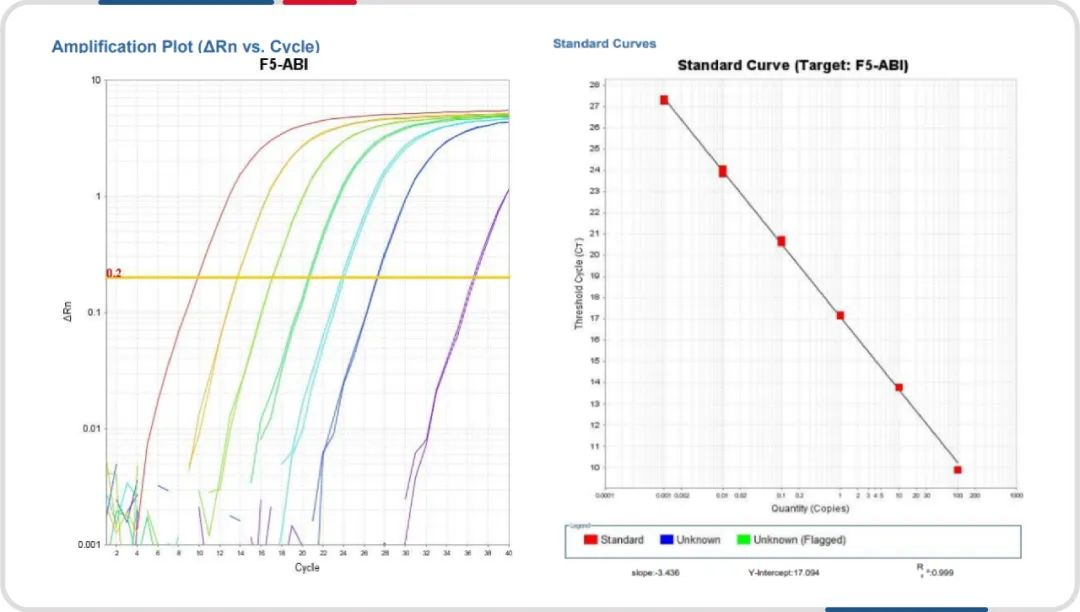
Standard Amplification Curve and Linearity Plot
Plasmid Residual DNA Detection Kit Product Information
1. HBP003305S 30T
2. HBP003305 100T











Please first Loginlater ~