Being Exquisite -- Interpreting the mRNA Purification Process
The reaction mixture of mRNA obtained by in vitro transcription (IVT) contains the needed mRNA outcomes, proteins (T7 RNA polymerase, inorganic pyrophosphatase), NTP, and impurities such as mRNA by-products formed in IVT (dsRNA, truncated RNA fragments). The presence of these impurities may affect the accurate quantification of mRNA, reduce its translation efficiency, or alter immunogenicity. Therefore, it is necessary to separate the target mRNA from the IVT mixture by purification technology to ensure its purity and integrity, and guarantee the safety and effectiveness of the product.
Common mRNA purification methods are lithium chloride (LiCl) precipitation, ammonium sulfate precipitation, magnetic bead purification, silica gel column membrane purification and chromatography purification. Among them, lithium chloride (LiCl) precipitation, magnetic bead purification and silica gel column membrane purification are relatively simple and easy to operate, but the purification efficiency is limited due to the volume. It makes them often used in scientific research and early research application scenarios. However, industrial production application applies chromatography purification method principally.
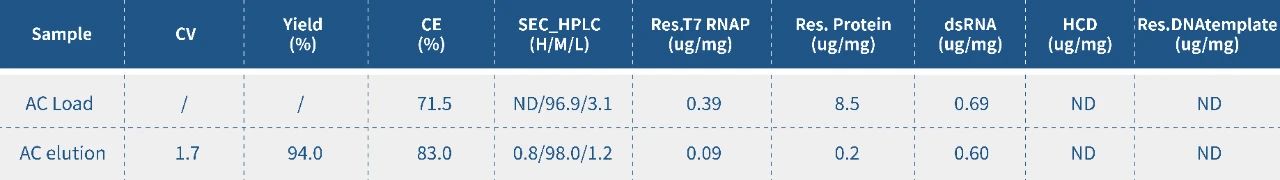
At present, popular industrial mRNA purification processes workflow is: TFF1-chromatograph-TFF2. In the case of high quality IVT products (high integrity, low by-products), the downstream purification process can be used to add protease K+TFF. Protease K, as an efficient broad-spectrum serine protease, can effectively remove protein impurities that bind to nucleic acids.
However, protease K itself has certain immunogenicity, which may affect mRNA vaccines safety and effectiveness. Therefore, in practical applications, how to balance the amount of protease K is particularly important.
How to balance the amount of protease K added?
The addition of the final concentration of protease K after IVT should be optimized, to make the protein impurities that bind to nucleic acid can be effectively removed when incubated at 37℃, mRNA integrity will not be affected. Hzymes has developed a kit for detecting protease K residues, and set quantitative detection indicators to ensure the safety and effectiveness of mRNA vaccines.
mRNA purification process development & mRNA purification kits
By using independently developed mRNA purification process platform, Hzymes provide customized production services for industrial-grade mRNA stock solution, and development services for mRNA purification process for different types of mRNA development.
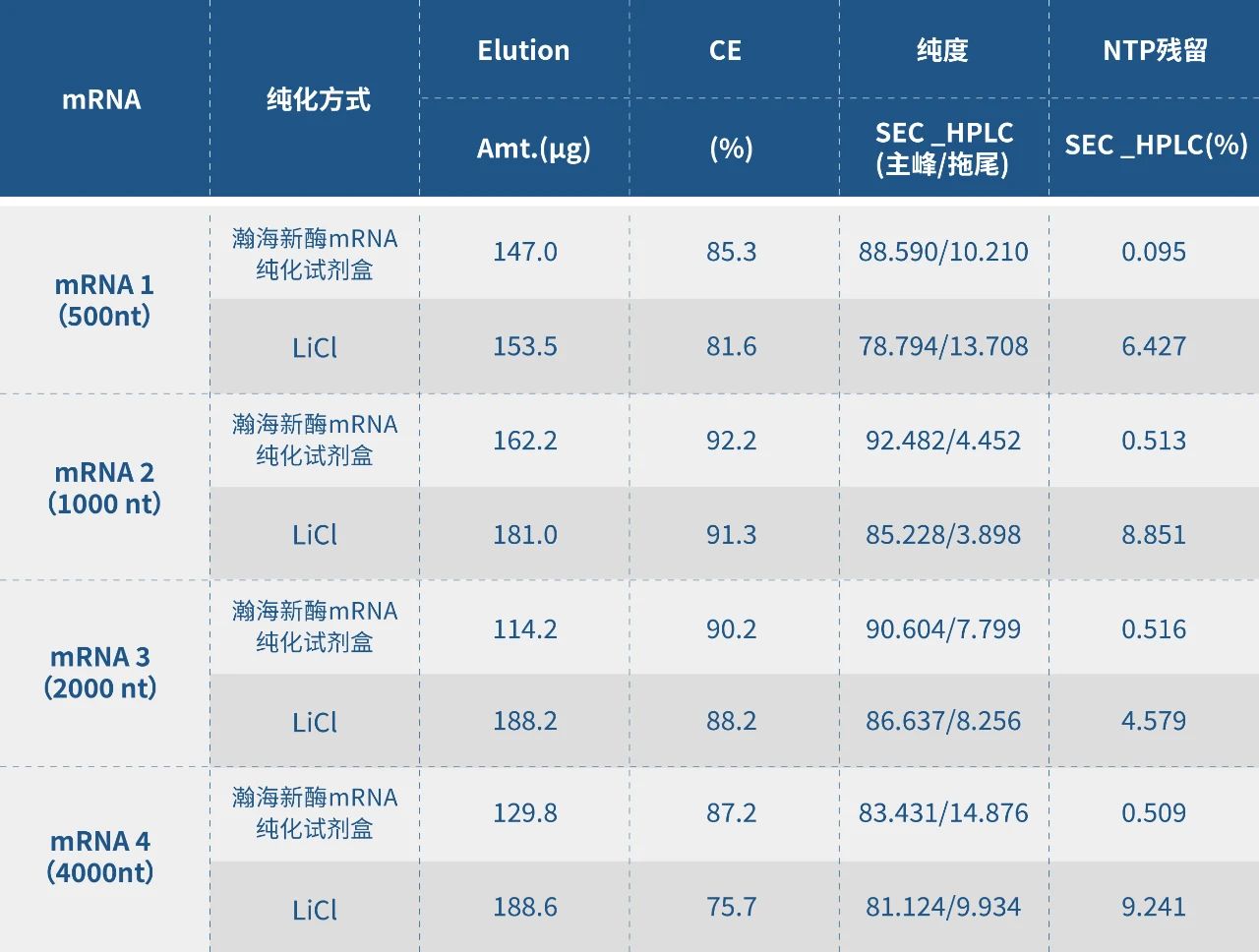
Meanwhile, Hzymes’ mRNA purification kit suit for scientific research and early research stage. Compared with the mainstream chromatography method at the industrial level, it is convenient in operation, no large equipment required and shortens process time. On the basis of ensuring convenient and fast operation, it also improves the integrity and rather competitive in removing impurities such as NTP.
Bibliography
[1]LiCl Precipitation for RNA Purification, Retrieved Jan 8, 2024,
[2]Product of BeyoMag™ RNA Clean Magnetic Beads, Retrieved Jan 8, 2024,
[3]Product of VAHTS RNA Clean Beads, Retrieved Jan 8, 2024,
[4]RNA Clean XP Performance and Data, Retrieved Jan 8, 2024,
[5]D Prazeres, T Schluep, C Coony, Preparative purification of supercoiled plasmid DNA using anion exchange chromatography, J Chromatogr A 806 (1998) 31–45.
[6]J Koubek, KF Lin, YR Chen, RP Cheng, JJT Huang, Strong anion-exchange fast performance liquid chromatography as a versatile tool for preparation and purification of RNA produced by in vitro transcription, RNA 19 (2013) 1449-1459.











Please first Loginlater ~