Considerable Breakthroughs! Announcement of the One-Minute Enveloping mRNA
Background
The COVID-19 has accelerated the development of mRNA vaccines. The development of lipid nanoparticle (LNP) delivery system and the maturation of large-scale preparation process enabled mRNA vaccines to move from concept to successful commercial application. LNP is the most mature and effective mRNA delivery vector at present, and the first two mRNA COVID-19 vaccines on the market are both delivered in LNP.
Traditional LNP Preparation Process
The delivery principle of mRNA-LNP: Cationic lipids are electrostatically complexed with negatively charged mRNA molecules to form a complex and improve the stability of mRNA molecules.
There are various preparation methods for mRNA-LNP, the microfluidic technology is relatively simple and fast, with high packaging efficiency, good uniformity and batch repeatability, which make it easy to achieve scale-up production.
In the early stage of research, a lot of target screening and sequence optimization are often required to determine the drug target, UTR and CDS sequences that meet the design requirements.
At present, the mRNA-LNP in vivo samples delivering have a large demand for initial encapsulation samples, and complex downstream ultrafiltration and purification processes are required after encapsulation. After purification, a variety of detection methods require high-precision instruments and tedious quality inspection steps, which delays the progress of mRNA sequence screening in the early research stage.
Considerable Breakthroughs!
Announcement of the One-Minute Enveloping mRNA
In response to the above sore points, Hzymes have launched a ready-to-use in vivo delivery LNP - Fast LNP (in vivo- System delivery).
1. Fast and easy, no equipment required, simple operation, no sample loss, initial sample required as low as μg.
2. Ethanol free in the component, high safety, no complex downstream ultrafiltration and purification process, no complex sample quality control process.
3. Ready-to-use LNP, efficient encapsulation scheme specially developed for RNA delivery in laboratory animals.
With Fast LNP, the cost and cycle of in vivo validation in animals can be greatly reduced. The high-precision, ultra-fast, low-cost and high-safety mRNA validation can be conducted directly across the cell level which make it widely applicable to mRNA, gRNA, saRNA and other RNA types.
mRNA Encapsulation in One Minute
Context of Use
01 In vivo evaluation of RNA drugs
02 In vivo high throughput screening of RNA sequences
03 mRNA vaccine and drug development
04 Efficiency analysis of gene editing
05 Animal immunity and antibody preparation
06 Trace RNA LNP Encapsulation
Advantages
- 1. Ready-to-use, simple mixing and no sample loss, 1μg mRNA can also be encapsulated.
- 2. Cost-effective, no encapsulation equipment required, east to use, saving cost.
- 3. Self-developed, exclusive lipids and formulations, no patent conflicts with Moderna and BNT/ Pfizer LNP formulations.
- 4. One-step rapid in vivo screening, direct expression verification at animal level, shorten the screening verification cycle.
- 5. Safety-guaranteed, high biosafety, no ethanol in the components.
- Applications
- Fast LNP goes beyond cell level validation and directly perform ultra-fast and low-cost in vivo validation. Hzymes provides in vivo validation based on the Fast LNP, directly output in vivo expression efficiently, and launch high-quality services with customized solutions.
Meet the rapid in vivo validation of multiple dosing modes
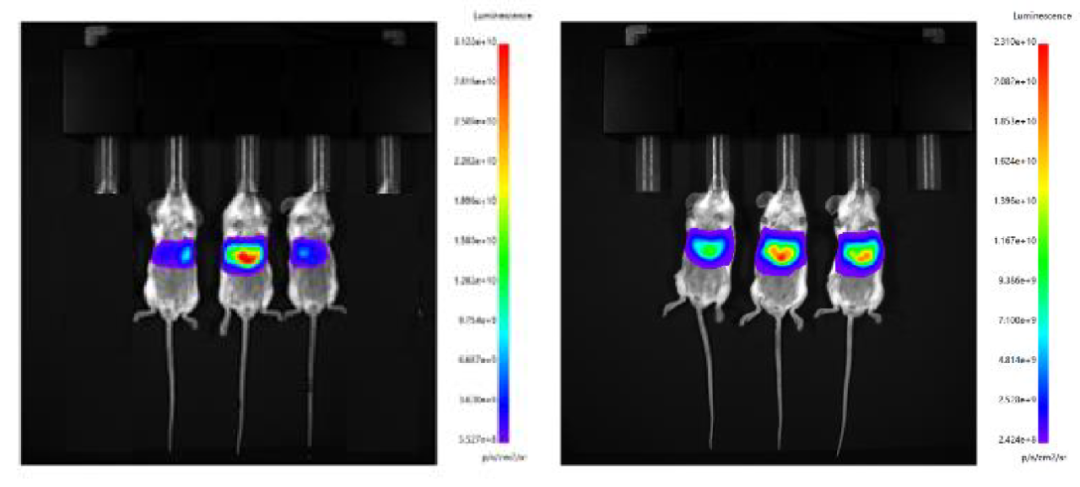
1. Intravenously, microfluid-controlled LNP and Fast LNP (in vivo) of Hzymes were administered intravenously, respectively.
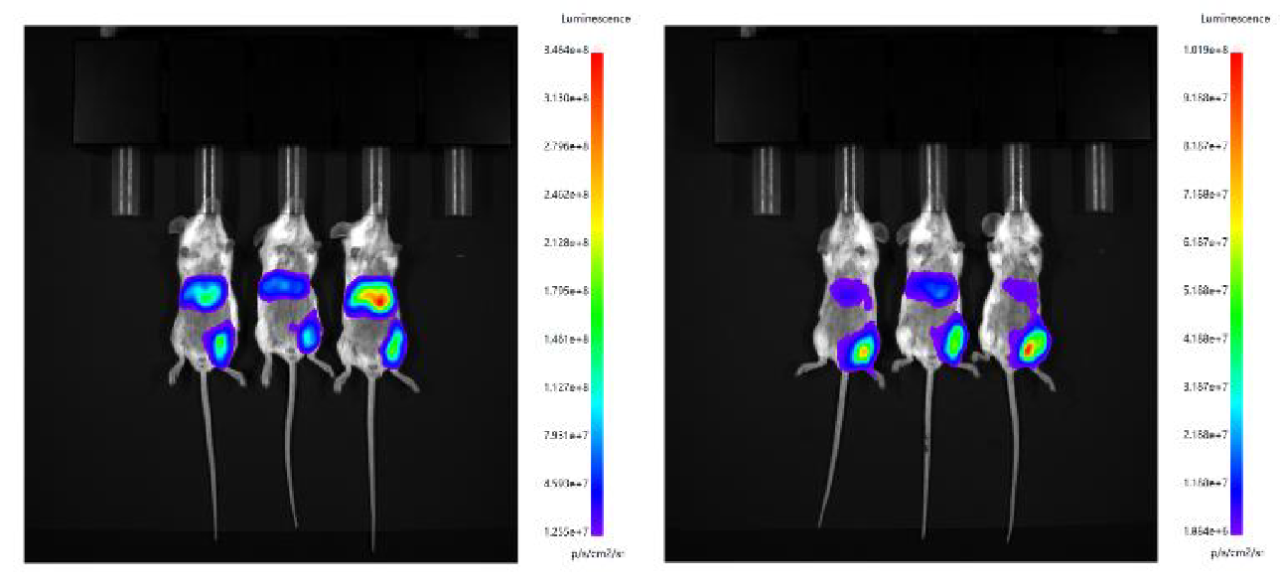
2. Intramuscular injection was performed by using microfluid-controlled LNP and Fast LNP (in vivo) of Hzymes, respectively.
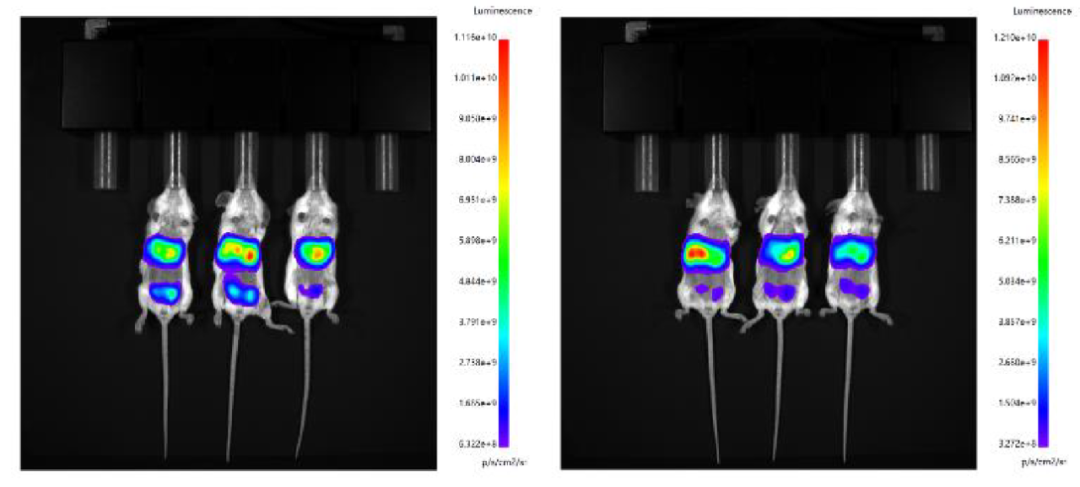
3. Intraperitoneal administration was performed by using microfluid-controlled LNP and Fast LNP (in vivo) of Hzymes, respectively.
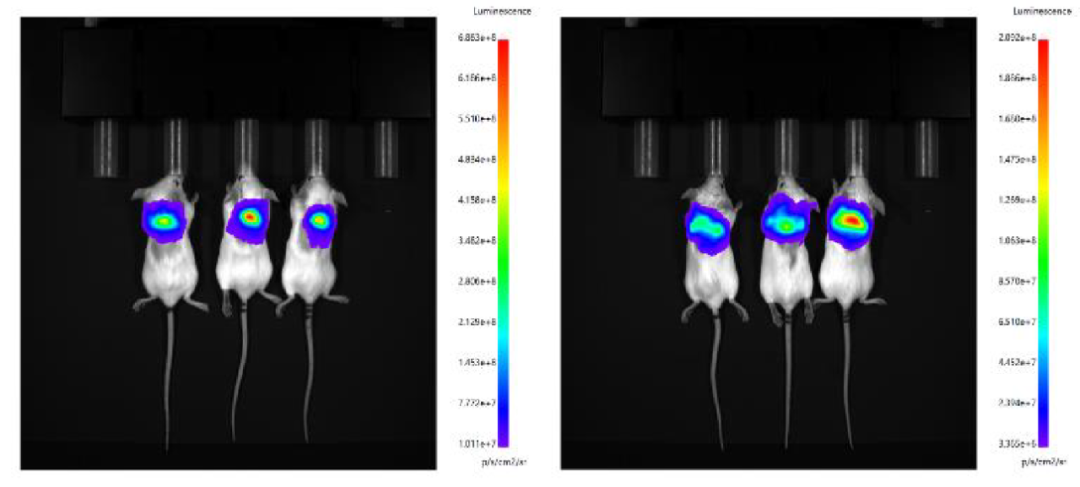
4. LNP prepared by microfluidic control and Fast LNP (in vivo) of Hzymes were administered subcutaneously, respectively.












Please first Loginlater ~